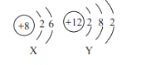��Ŀ����
����Ŀ���й���ȫ���һ��ʵ���ں�������ȼ�����Կ����л�������ȶ������Ĺ��ҡ�����ȼ�����Ǽ����ˮ�ڵ��¡���ѹ�������γɵ�ˮ���CH4nH2O��
��1�������ڿ�����ȼ�յĻ�ѧ����ʽ��_____��
��2��������Ƴɺϳ�����CO��H2�������Ƴɼ״���CH3OH�����������湩Ӧ���ŵ�ȼ�ͣ��ɺϳ����Ƽ״��ķ�Ӧ����ʽΪ��CO+2H2![]() CH3OH���ɼ����Ƴɺϳ��������ַ�����
CH3OH���ɼ����Ƴɺϳ��������ַ�����
��CH4+H2O![]() CO+3H2 ��2CH4+O2
CO+3H2 ��2CH4+O2![]() 2CO+4H2
2CO+4H2
��ԭ����ȽǶȱȽϷ����ٺ͢ڣ����������ںϳɼ״��ķ�����_____������ţ���
���𰸡�CH4+2O2![]() CO2+2H2O ��
CO2+2H2O ��
��������
��1������������е������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽ�ǣ�![]() ��
��
��2��ѡ��CH4����ȫȼ���ƺϳ���ʱ���ų�������ͬʱ�õ���CO��H2���ʵ���֮��Ϊ1��2����ǡ����ȫ��Ӧ�ϳɼ״�����ѡ�ڡ�

 ����5��2���ϵ�д�
����5��2���ϵ�д�����Ŀ��Ϊ�˲ⶨijͭп�Ͻ���п������������ijͬѧ���øúϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
��һ�� | �ڶ��� | ������ | |
��ȡ�Ͻ������/g | 25 | 25 | 50 |
����ϡ���������/g | 120 | 160 | 100 |
��������������/g | 0.4 | 0.4 | 0.4 |
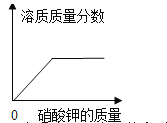
��1�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������Ϊ_____ʱ�������Ͻ��е�п��ϡ�����е�������ȫ��Ӧ��
��2���Լ����ͭп�Ͻ���п����������_____��
��3������ϡ������Һ�����ʵ���������_____��
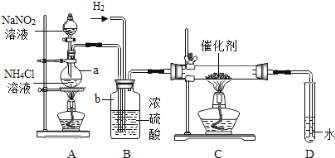
����Ŀ����ͼ�Dzⶨij���������Fe2O3����������ʵ��װ�ã�
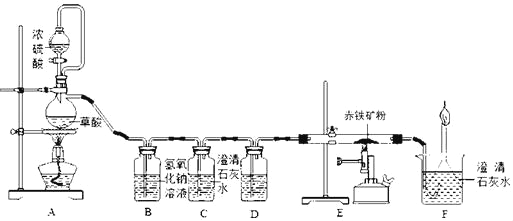
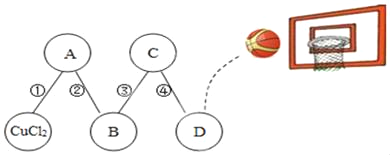
��1��Aװ�õ���������ȡһ����̼����Ӧ�Ļ�ѧ����ʽΪH2C2O4 ��CO2��+CO��+H2O�����ݷ�Ӧװ���жϣ���ѧ����ʽ�л�ȱ�ٵ�������_____��
��2��Cװ�õ�������_____��Dװ����ҩƷ��������_____��Eװ���з�����Ӧ�Ļ�ѧ����ʽΪ_____��F����β��ȼ�յ���Ŀ����_____��
��3��ʵ�������Ӧ���ȵ�ȼA������E���ļ���װ�ã�_____��
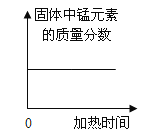
��4��ʵ���м�¼���������±����ݴ˼��������������������������Ϊ_____��
���������� | �����ܺ�ҩƷ��Ӧǰ���� | �����ܺ�ҩƷ��Ӧ������ |
71.8g | 81.8g | 79.4�� |
��5����ͬѧ������Ը���Fװ�������ӵ����������������������������������ͬ��˿�����˵����Ĺ۵��������_____��