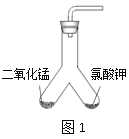
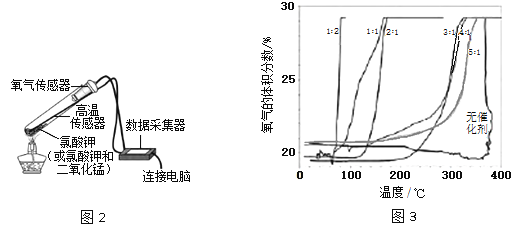
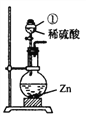
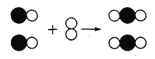��Ŀ����
����Ŀ����֪ij����X�����������Ϣ���� ������Ҫ����Ļ�ѧ�ɷ���X2O3���� ����Ҫͨ���Ȼ�ԭ��ұ�����ɣ��� ���������λ�ڽ���֮�ס�
(1) �ݴ��ƶ�X��_____________(��ѡ����ĸ)�� A���� B���� C���� D��ͭ
(2)�ڸ�¯���ú�X2O3�Ŀ���ұ���ý�����ԭ����____________(�û�ѧ����ʽ��ʾ)��
(3)ͬѧ�����������ʵ�鷽���ⶨ�ÿ�����X2O3����������(װ�����������ã������е����ʲ��μӷ�Ӧ�����������Ʒ�е�X2O3��ȫ��Ӧ)��
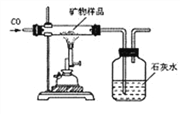
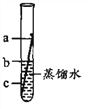
��ȡ������Ʒ����������Ʒ��������
�ڲ����Ӧǰ���ƿ��ƿ��������������
�۲����Ӧ����ƿ��ƿ�������������� �ܼ���ó�������Ʒ��x2O3������������
a. ����Ϊ����ʵ�鷽��___________(����һ����������һ����)��ȷ���������X2O3������������������____________________________ .
b. ���ı�װ�ú�ҩƷ���㻹����ͨ���ⶨ��Щ���ݣ���ͨ������ó�������X2O3������������_________________________________��
c. �ӻ����Ƕȿ�����װ�õIJ���֮����____________________��
���𰸡� B X2O3+3CO ���� 2X+3CO2(��Fe2O3+3CO���� 2Fe+3CO2) a��һ�� ���ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ����(��ʯ��ˮ�������տ����еĶ�����̼) �ֱ�����Ӧǰ�����ܺͿ�����Ʒ������������Ӧ�����ܺ�ʣ�����������(������Ӧ��������ʣ����������) ��β������װ��
��������(1) �ݴ��ƶ�X��B���������������λ�ڽ���֮�ס�(2)�ڸ�¯���ú�X2O3�Ŀ���ұ���ý�����ԭ���ǡ�X2O3+3CO![]() 2X+3CO2(��Fe2O3+3CO
2X+3CO2(��Fe2O3+3CO![]() 2Fe+3CO2)��(3) �ⶨ�ÿ�����X2O3������������a. ��ʵ�鷽����һ����ȷ���������X2O3���������������������ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ����(��ʯ��ˮ�������տ����еĶ�����̼)��b. ���ı�װ�ú�ҩƷ��������ͨ���ⶨ��Ӧǰ�����ܺͿ�����Ʒ������������Ӧ�����ܺ�ʣ�����������(������Ӧ��������ʣ����������)����ͨ������ó�������X2O3������������c. �ӻ����Ƕȿ�����װ�õIJ���֮������β������װ�ã�β����Ⱦ������
2Fe+3CO2)��(3) �ⶨ�ÿ�����X2O3������������a. ��ʵ�鷽����һ����ȷ���������X2O3���������������������ɵĶ�����̼���岻һ����ʯ��ˮ��ȫ����(��ʯ��ˮ�������տ����еĶ�����̼)��b. ���ı�װ�ú�ҩƷ��������ͨ���ⶨ��Ӧǰ�����ܺͿ�����Ʒ������������Ӧ�����ܺ�ʣ�����������(������Ӧ��������ʣ����������)����ͨ������ó�������X2O3������������c. �ӻ����Ƕȿ�����װ�õIJ���֮������β������װ�ã�β����Ⱦ������