��Ŀ����
����Ŀ��ˮ������֮Դ��Ҳ�ǹ�ũҵ��������ȱ�ٵ����ʣ��������úͱ���ˮ��Դ�������岻�ݴǵ����Ρ�
��1������������˵������������_____������ţ�
���ϸ���ƻ��ʺ�ũҩ��ʹ��
�ڻ���̽����ˮ�������¼���
�������п�ͨ����н���ˮ��Ӳ��
�ܺ�ˮ�����������˴�����õ���ˮ
��2��ijͬѧ����Ͳ��ȡ����ˮ�Ĺ����и��Ӷ�����������ȡˮ�������_____���ƫ����ƫС��������Ӱ�족����
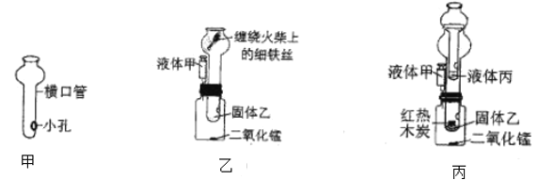
��3���ھ��꼶�̲ĵ��ˮ��ʵ���У����������ų�������֮����������_____��ͨ�����ˮ��ʵ��˵��ˮ����_____��
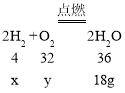
��4��������������Ӧ�ֿ�������ˮ����һ���ܱ����������������������Ļ������20g����ȼ���������18g��ˮ����ԭ��������������������������ȿ�����_____��_____��
���𰸡��� ƫС 1��2 ��Ԫ�غ���Ԫ����� 1��4 1��9
��������
��1�����ϸ���ƻ��ʺ�ũҩ��ʹ�ã������ڱ���ˮ��Դ������������
�ڻ���̽����ˮ�������¼����������ڱ���ˮ��Դ������������
����п�ʹˮ�и�þ���ӳ����������п�ͨ����н���ˮ��Ӳ�ȣ�˵����ȷ��
�ܺ�ˮ�����������˴�����õ��IJ��Ǵ�ˮ���õ���ˮ���кܶ���������ʣ�˵������
����ܡ�
��2������Ͳ��ȡ����ˮ�Ĺ����и��Ӷ�����������ȡˮ�������ƫС��
��3�����ˮ��ʵ���У��������������������������һ�������������ų�������֮����������1��2��ͨ�����ˮ��ʵ��˵��ˮ������Ԫ�غ���Ԫ����ɵġ�
��4����μӷ�Ӧ��Ӧ�������������������ֱ�Ϊx��y����
![]()
���x=2g��y=16g��
����ʣ������Ϊ������������������Ϊ2g+��20g-18g��=4g���������������4g������16g��������������������������1��4��
����ʣ������Ϊ������������������Ϊ16g+��20g-18g��=18g���������������2g������18g��������������������������Ϊ1��9��

����Ŀ��Cu��Zn�ĺϽ��Ϊ��ͭ���������ĵ����Ժ���ʴ�ԣ��������������������ij��ѧ��ȤС���ͬѧΪ�˲ⶨij��ͭ����ɣ�ȡ20g�û�ͭ��Ʒ���ձ��У������з�5�μ�����ͬ��������������ϡ���ᣬʹ֮��ַ�Ӧ��ÿ������ϡ�����������ʣ������������¼�������
����ϡ�����������g�� | ��ַ�Ӧ��ʣ������������g�� | |
��1�� | 20 | 17.4 |
��2�� | 20 | 14.8 |
��3�� | 20 | 12.2 |
��4�� | 20 | 12 |
��5�� | 20 | m |
�Իش��������⣺
��1������������m��ֵΪ_____��
��2����ͭ��Ʒ��п������Ϊ_____��
��3������ϡ���������������������_____��
����Ŀ��ij��ȤС��̽��Ӱ��H2O2�ֽ����ʵ����ء��������й�̽�����̵�����ʵ�飺
��1����ȤС��������ͼaװ��̽��Ӱ��H2O2�ֽ����ʵ����أ�ʵ����Ʒ������±����������ɣ����пվ�����������
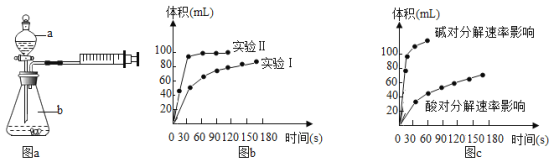
ʵ���� | ʵ��Ŀ�� | t�� | ���� | Ũ�� |
ʵ��� | ��ʵ����� | 25 | 3��FeCl3��Һ | 10ml 2%H2O2 |
ʵ��� | _________ | 25 | _________ | 10ml 5%H2O2 |
�ٷ�Ӧ�Ļ�ѧ����ʽΪ_____________��
��ʵ��ó���������ͼb����ʵ��ó������ݿ�֪��Ũ��Խ��H2O2�ֽ�����______________��
��2����ȤС����̽�����ᡢ���H2O2�ֽ����ʵ����أ�ʵ��ó���������ͼc����������ͬ�����£�______�����������������������ڵ�����·ų��������ʽϿ죻��ó��˽��۵�������____________��