��Ŀ����
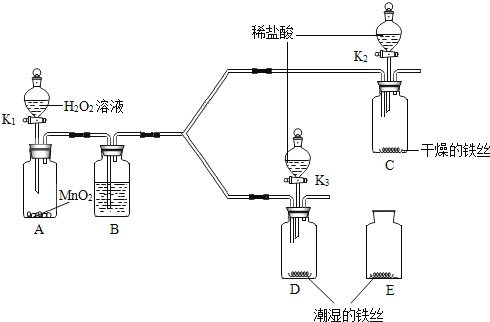
����Ŀ����ѧ��Ӧ�ڷ��λ�����Ⱦ�а�������Ҫ��ɫ��ij���������е�SO2���������·�ʽ��������ʽһ��2SO2+O2+2CaO=2CaSO4 ��ʽ����2SO2+O2+4NaOH=2Na2SO4+2H2O �Լ��㣺 (��֪��CaSO4�ļ۸�Ϊ700Ԫ/�֣���Է�������ΪCaSO4 136 Na2SO4 142)��
(1)CaSO4��������Ԫ�ص�������������������ϵ��������Ԫ������Ԫ�غ�_________��
(2)���÷�ʽһ����9.6tSO2�����ò�Ʒ��ֵ___________Ԫ?
(3)���÷�ʽ������9.61t SO2���պ���ȥ50tһ��Ũ�ȵ�NaOH��Һ����������Һ��������������________(���ս����ȷ��0.1%)��
���𰸡� ��Ԫ�� 14280 34.4%
�����������⿼���˸��ݻ�ѧʽ������ݻ�ѧ����ʽ���㡣
��1����CaSO4������Ԫ������Ԫ�ص�������=32����16��4��=1��2��������Ԫ�غ���Ԫ��������������������ϵ��
��2����ʽһ���裺���ɵ�����Ƶ�����Ϊx
2SO2+O2+2CaO=2CaSO4
128 272
9.6t x
![]() x=20.4t
x=20.4t
���ò�Ʒ��ֵ700Ԫ/t��20.4t=14280Ԫ
��3����ʽ�����������ɵ������Ƶ�����Ϊy�����ĵ���������Ϊz��
2SO2+O2+4NaOH=2Na2SO4+2H2O
128 32 284
9.6t z y
![]() y=21.3t
y=21.3t
![]() z=2.4t
z=2.4t
������Һ��������������=![]() ��100%��34.4%��
��100%��34.4%��
�𣺣�1��CaSO4��������Ԫ�ص�������������������ϵ��������Ԫ������Ԫ�غ� ��Ԫ�ء�
��2�����÷�ʽһ����9.6tSO2�����ò�Ʒ��ֵ14280Ԫ��
��3�����÷�ʽ������9.6tSO2���պ���ȥ50tһ��Ũ�ȵ�NaOH��Һ��������Һ��������������Ϊ34.4%��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������ʵ����ѡ�Լ��Ͳ�����������ȷ���ǣ�������
ѡ�� | ʵ��Ŀ�� | ��ѡ�Լ������� |
A | ��ȥ | ��ͨ�� |
B | ��ȥ | ���������� |
C | ���� | �ֱ��������ˮ�۲����� |
D | ��ȥ����������������ͭ��ĩ | ����������ˮ�ܽ⡢���ˡ�ϴ�Ӹ��� |
A. AB. BC. CD. D
����Ŀ��С��ͬѧ�����Լ���ͭ���۾�������������ɫ��ͭ�⡣Ϊ��Ū��ͭ�̵���ɺ�ͭ��������ͭ�̵�������С�������ǵĻ�ѧʵ��С�����������̽��ʵ�飺
��һ��̽��ͭ�̵���ɡ�
���������ϣ���ͭ�⡱��Ҫ�ɷ���Cu2(OH)2CO3���׳�ͭ�̣���ͭ�������ֽ⡣
��ʵ����ƣ�
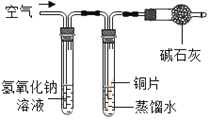
��С��ͬѧ��ͭ���۾�����ȡ�������ĸ���ͭ�̣���ͼ��ʾװ�ý���ʵ�顣��ʵ������У��۲쵽���Թܿ���ˮ����֣�˵����ͭ���к���_________Ԫ�أ�����ʯ��ˮ����ǣ�˵����ͭ���к���________Ԫ�ء�
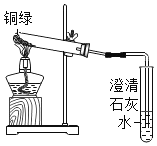
��С��ȡ�������Թ��ڷ�Ӧ���ʣ���������һ֧�Թ��У�����ϡ���ᣬ��ַ�Ӧ����Һ����ɫ��˵��ͭ���к���___________Ԫ�ء�
��ʵ����ۣ���______________________________________________________________________________��
������̽��ͭ�����������
���������ϣ�1ͭ�����Ȼ�ֽ�����������
��д��ͭ�̷ֽ�Ļ�ѧ����ʽΪ_______________________________________________________________��
2��ʯ����Ҫ�ɷ����������ƺ������ƹ���Ļ���ʵ���ҳ�������������Һ���ն�����̼���塢���ü�ʯ�ҳ�ȥ������̼�����ˮ������
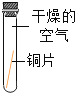
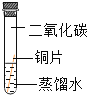
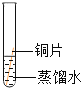
��ʵ����ƣ�����α���������ʴ������̽����ʵ�飬Сƽ����ˡ�ͭƬ��ʴ������̽����ʵ�飬ʵ������ͼ��ʾ����С��ͭƬ�ֱ���ͼ��ʾ����һ���£��۲��������£�
ʵ��װ�� |
|
|
|
|
ʵ������ | ͭƬ������ | ͭƬ������ | ͭƬ������ | ͭƬ������ˮ�渽����ʴ�����ص� |
��ʵ����ۣ���ͭ������ͭ��������___________��__________�����ʹ�ͬ���õĽ����
����˼Ӧ�ã���д����ֹ����ͭ�Ƚ�����Ʒ��������ֲ�ͬԭ���ķ���__________________________________________________________________________________________________��