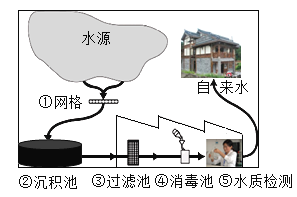
��Ŀ����
����Ŀ���Ȼ�泥�NH4Cl������Ҫ�ĵ��ʣ������ƣ�Na2SO4��������ֽ����Ⱦ��ϡ�ͼ���ҽҩƷ�ȵ���Ҫԭ�ϣ�����Na2SO4��NH4Cl���ܽ�����ش��������⡣
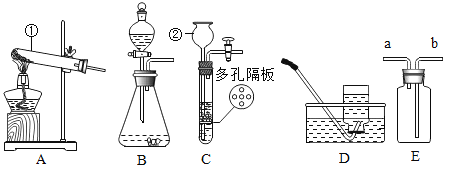
��1�����д�ʩ�У�����ʹ40��ı�����������Һ�������������____��
A �����ܼ��� B �����ܼ� C �����¶� D �����¶�
��2����������70��ı���Na2SO4��Һ����Һ������������29g��������Na2SO4���������Ϊ_____g��
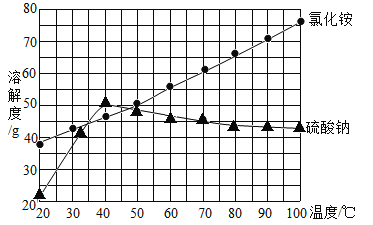
��3��70��ʱ��60gNH4Cl��40gNa2SO4��ȫ�ܽ���100g����ˮ�У���ȴ��40���_____����С���û�С���NH4Cl��������ʱ��Һ��������NH4Cl_____������ڡ���С�ڡ���Na2SO4��
��4��t2��ʱ��B���ʵ��ܽ��Ϊ40g/100gˮ����150gˮ��Ͷ��_____g��B���ʣ���Һ���ﵽ���͡�
���𰸡�B 9 �� ���� 60
��������
��1�������Ƶ��ܽ����40��ʱ�����ʹ40��ı�����������Һ����������ǣ�
A���ܼ�Խ�٣����ʵ��ܽ���Խ�٣������ܼ���������������Һ���������壻��ѡ�����
B���ܼ�Խ�࣬���ʵ��ܽ���Խ�࣬�����ܼ���������������Һ�����������壻��ѡ����ȷ��
C�������¶ȣ��������ܽ�Ƚ��ͣ���������Һ���������壻��ѡ�����
D�������¶ȣ��������ܽ�Ƚ��ͣ�������������Һ���������壻��ѡ�����
��ѡ��B��
��2��70��ʱ�������Ƶ��ܽ��Ϊ45g����������70��ı���Na2SO4��Һ����Һ������������29g��
������Na2SO4���������Ϊx��
![]() x=9g
x=9g
���������ƹ�������Ϊ9g��
��3��70��ʱ��60gNH4Cl��40gNa2SO4��ȫ�ܽ���100g����ˮ�У���ȴ��40���ʱ�����Ƶ��ܽ�ȴ���40g���Ȼ�淋��ܽ��С��60g������NH4Cl��������ʱ��Һ��������NH4Cl����Na2SO4����Ϊ40���Ȼ����Һ�е�������Ȼ����40g������������Һ�е�������Ȼ��40g��
��4��t2��ʱ��B���ʵ��ܽ��Ϊ40g/100gˮ����100gˮ������ܽ�40gB���ʣ�50gˮ������ܽ�20gB���ʣ�����150gˮ��Ͷ��60g��B���ʣ���Һ���ﵽ���͡�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
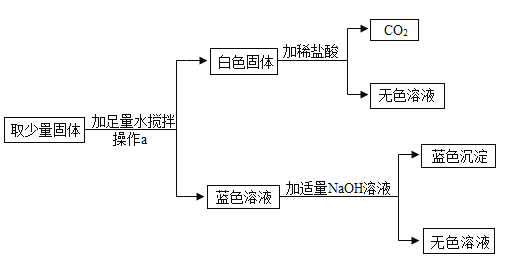
�����ܿ����ϵ�д�����Ŀ��ijͬѧ���֣��ϸ�����ʵ���õ�NaOH��Һ�����˸�ƿ�ǣ����ڸ���Һ�Ƿ���ʣ�ͬѧ�ǿ�ʼʵ��̽����
��������裩
���루1��������Һû�б��ʣ�ΪNaOH��Һ��
���루2��������Һȫ�����ʣ�ΪNa2CO3��Һ��
���루3��������Һ���ֱ��ʣ�ΪNaOH��Na2CO3�Ļ����Һ��
���������ϣ�Na2CO3��Һ�ʼ���
����Ʒ��������������±�����ͬѧ̽�ֵ���Ʒ���
ʵ����� | ���ܳ��ֵ���������� | ͬѧ���� | |
��1�� |
| ����Һ��죬����루2�������� | ��2��ͬѧ��Ϊ���˷������۲���ȷ�������ǣ�__�� |
��2�� |
| ��������ɫ��������Ӧ����ʽ�ǣ�_____ ����루1���������� | ��3��ͬѧ��Ϊ���˷���������ȷ�����루2�����Dz��루3�������� |
��3�� |
| ����Һ����죬����루2������������Һ��죬����루3�������� | ��1��ͬѧ��Ϊ���������Ҳ�ܴﵽʵ��Ŀ�ģ����IJ��������ǣ�_____ |
��̽��ʵ�飩�ۺϸ�С��ķ�������ʵ�飮
�������뷴˼����1��NaOH��Һ���������_____��Ӧ�����ʣ�Ϊ��ֹ���ʣ����Ա���ʱҪ_____��
��2���ڶ���ʵ���У����������ķ���ʽΪ��_____��
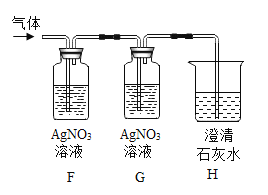
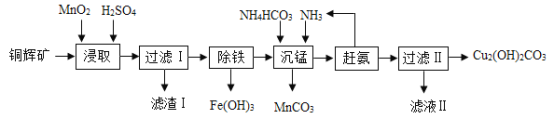
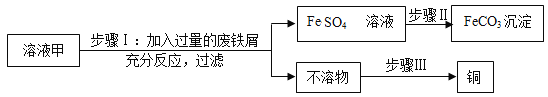
����Ŀ��̼����泥�NH4HCO3�����׳ơ�̼李�����ũ�峣�õĵ��ʡ�NH4HCO3������ˮ���������ֽ⣬�����������ʣ�����⣬�����������������£�
������ | ������� |
�����һ�� | ��ɫ��ζ��Һ�壬�������ܼ� |
��������� | ��ʹ����ʯ��ˮ����ǵ����塣 |
��������� | �д̼�����ζ�����壬ˮ��Һ�ʼ��ԡ� |
��1����д��̼��������ȷֽ�Ļ�ѧ����ʽ��_____��
��2����Ԥ��̼����淋Ļ�ѧ���ʣ�˵��Ԥ������ݣ������ʵ����֤��
��ѡ�Լ��ǣ�̼����立�ĩ����ʯ�ҡ�ʳ�Ρ�ϡ���ᡢ��ɫʯ����Һ��̿�ۡ�þ��������ͭ��
Ԥ�� | Ԥ������� | ��֤Ԥ���ʵ�������Ԥ������ |
���ܹ������෴Ӧ | ̼������������̼��������� | ȡ����̼����立�ĩ���Թ��У�����������_____������۲쵽_____����Ԥ������� |
���ܹ���_____����������𣩷�Ӧ | _____ | ȡ����̼����立�ĩ_____�����_____����Ԥ������� |