��Ŀ����
��10�֣�����A ~ F�dz��л�ѧ�е�����ʵ��װ�ã��밴Ҫ����գ�

С��1:Aʵ���Թ�2�в����������� ����Ӧ�Ļ�ѧ����ʽΪ
С��2:Cʵ�����ձ��۵������� ��
С��3:Dʵ��˵�����������Լռ������ ����Ӧ�Ļ�ѧ����ʽΪ
С��4:����Eװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ ��
С��5:����ʵ������в���ȷ���� ������ĸ����
С��6:Fʵ���У�����ı仯������� ���� ������С����д������������Ļ�ѧ����ʽ ��

С��1:Aʵ���Թ�2�в����������� ����Ӧ�Ļ�ѧ����ʽΪ
С��2:Cʵ�����ձ��۵������� ��
С��3:Dʵ��˵�����������Լռ������ ����Ӧ�Ļ�ѧ����ʽΪ
С��4:����Eװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ ��
С��5:����ʵ������в���ȷ���� ������ĸ����
С��6:Fʵ���У�����ı仯������� ���� ������С����д������������Ļ�ѧ����ʽ ��
��1:������H2�� 2H2O
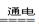 2H2��+O2��
2H2��+O2�� С��2:���ԱȻ�Ա�ʵ�����Ʊ�����
��3:1/5 4P + 5O2 ="==" 2P2O5
С��4:Ũ����
��5:B
С��6:��� ��С CO2 + 2NaOH ="=" Na2CO3 + H2O
С��1:���ݵ��ˮ��ʵ���������Դ�����������Թܵõ�������Ϊ���������Դ�����������Թ��ڵõ�������Ϊ����������������������Ϊ2��1��
С��2:�ձ����ڷ�̪�����백�����ӽӴ������ᷢ����ɫ�仯������Ϊ�Աȣ�
С��3:Dʵ��ɹ۲쵽������ƿ�ڵ�ˮԼռ�������1/5 ˵�����������������Լ����������1 /5 ����
С��4:װ��E��װ���������ˮ�Ե�Ũ���ᣬ������ȥ����������ˮ�����ã�
С��5:ϡ��Ũ����ʱ��Ҫ���Ũ���ᵹ��ˮ�У���ֹҺ�彦��
С��6:���������������Һ����ƿ�ڶ�����̼��ʹƿ��ѹǿ��С���������ͨ���������ͣ��������ϡ����������̼���Ʒų�������̼ʹƿ��ѹǿ����ʹ���������ͨ���͵����彥������

��ϰ��ϵ�д�
�����Ŀ









