��Ŀ����
����Ŀ�����������������������������أ��μ����ս�����֪ʶ�dz���Ҫ��
��1��������;�����ý��������Ե���______������ĸ����
A������ B������ָ C���Ͻ����Ŵ� D����������
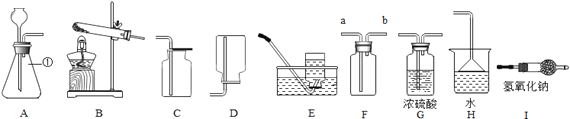
��2����ͼ�ǻ�ѧС��̽��������ʴ������ʵ�飬Ҫ�ﵽʵ��Ŀ�Ļ��貹��__________ʵ�飿
��3����ʵ���������������ͼװ���Ƶ�����
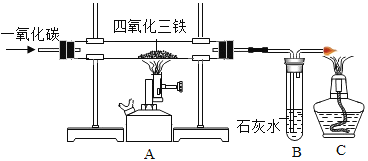
��A������������������Ӧ�Ļ�ѧ����Ϊ________________ ��
��B���ɹ۲쵽��������____________��C���ƾ��Ƶ�������_____________��
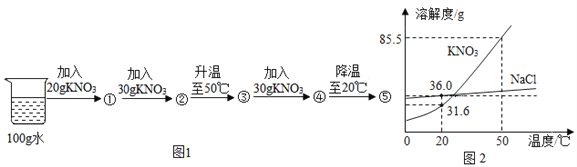
��4����һ��������������������������ͭ�Ļ����Һ�м���һ������п����Һ���������п��������ϵ��ͼ����ش�
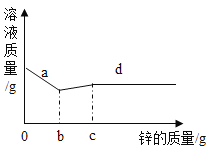
��д��a����������Ӧ�Ļ�ѧ����ʽ__________��
��d ���Ӧ����Һ�еĽ�����������Щ__________��
��5��Ϊ�˲ⶨͭп�Ͻ���Ʒ��п�ĺ�������ȡ��Ʒ20g���ձ��У������м���100gϡ������ǡ����ȫ��Ӧ����Ӧ���ձ������ʵ�������Ϊ119.6g������㣺��Ʒ��п����������__________��
���𰸡�A ���������ڸ���Ŀ����в�����ʵ�� Fe3O4+4CO![]() 3Fe+4CO2 ����ʯ��ˮ����� ��ȼ�����һ����̼��������Ⱦ���� Zn+2AgNO3=Zn(NO3)2+2Ag Al3+��Zn2+ 65%
3Fe+4CO2 ����ʯ��ˮ����� ��ȼ�����һ����̼��������Ⱦ���� Zn+2AgNO3=Zn(NO3)2+2Ag Al3+��Zn2+ 65%
��������
��1�����������������ý����ĵ����ԣ���ѡ��A��
��2������������ˮ����������ʱ����������������ʵ��������ֻ��ˮ�Ӵ�������ˮ������ͬʱ�Ӵ���ʵ�飬�����Ҫ���������ڸ���Ŀ����в�����ʵ�飬����ڸ���Ŀ����в�����ʵ�飻
��3��A����������������������һ����̼�ڸ����»��������Ͷ�����̼��ѧ����ʽΪFe3O4+4CO![]() 3Fe+4CO2��������̼ͨ�����ʯ��ˮ�����������������̼��Ƴ�����ˮ������ʯ��ˮ�����ǣ�һ����̼���ж��������Ⱦ����������β���������þƾ��ƽ�һ����̼��ȼ�����ɶ�����̼�����Ա���һ����̼��Ⱦ���������Fe3O4+4CO
3Fe+4CO2��������̼ͨ�����ʯ��ˮ�����������������̼��Ƴ�����ˮ������ʯ��ˮ�����ǣ�һ����̼���ж��������Ⱦ����������β���������þƾ��ƽ�һ����̼��ȼ�����ɶ�����̼�����Ա���һ����̼��Ⱦ���������Fe3O4+4CO![]() 3Fe+4CO2������ʯ��ˮ����ǣ���ȼ�����һ����̼��������Ⱦ������
3Fe+4CO2������ʯ��ˮ����ǣ���ȼ�����һ����̼��������Ⱦ������
��4����һ��������������������������ͭ�Ļ����Һ�м���һ������п����Ϊ�������Al��Zn��Cu��Ag��п�����������Ӧ��������п��������ѧ����ʽΪ��Zn+2AgNO3=2Ag+Zn(NO3)2�÷�Ӧ��Ӧ65������п����216�����������÷�Ӧ�����������ӣ���Һ������С��Ȼ���ٺ�����ͭ��Ӧ��������п��ͭ��ѧ����ʽΪZn+Cu(NO3)2=Cu+Zn(NO3)2�÷�Ӧ����65������п����64������ͭ������������С����Һ�������ӣ�
��a �㴦����Һ�������ϼ��ٵ������ϣ�˵��п��������������Ӧ����������Һ����ת��Ϊ����п����Һ�������ϼ������Է�����Ӧ�Ļ�ѧ����ʽΪZn+2AgNO3=2Ag+Zn(NO3)2���Zn+2AgNO3=2Ag+Zn(NO3)2��
�� d �㴦����Һ�����������ӵ���ĩ�ˣ�˵��п�ٺ�����ͭ��Ӧ����ǡ����ȫ��Ӧ����ʱ��Һ����Ϊ������������п������������ΪAl3+��Zn2+���Al3+��Zn2+��
��5�����������غ㷴Ӧ������������20g+100g-119.6g=0.4g����п������Ϊx��

�������������
![]()
�����������65%��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ����ͼ��ʾ����ϸ��˿�ֱ���ڳ��������������ļ���ƿ�н���ʵ�顣
ʵ�� | �� | �� |
����ƿ�е����� | ���� | ���� |
��ͨ��Դ���Ⱥ�۲쵽������ | ��˿���ȣ���ȼ�� | ����ȼ�գ��������� |
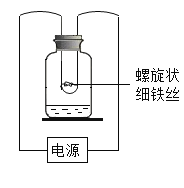
��1��ʵ����У���Ӧ���к�ɫ�������ɣ�����ƿ��ˮ��������_____________��
��2����ͨ��Դ�ԱȢ�ʵ��������˵��_____________��
��3��������ʵ���У���֤����ȼ��ȼ����Ҫ�¶ȴﵽ�Ż���������_____________��
����Ŀ��ѧϰ�˶������̶Թ��������д����õ�֪ʶ��ijͬѧ�룺����ͭ�ܷ������ƶ������̵Ĵ��������أ����ǽ���������̽����
�����룩������ͭ���Ǵ�����Ҳ�����뷴Ӧ����Ӧǰ�������ͻ�ѧ���ʲ��䣻
������ͭ���뷴Ӧ������������Ӧǰ�������ͻ�ѧ���ʷ����˸ı䣻
������ͭ�Ƿ�Ӧ�Ĵ�������Ӧǰ��_____��_____���䡣
��ʵ�飩����ƽ����0.2g����ͭ��ȡ5mL 5%�Ĺ���������Һ���Թ��У���������ʵ�飺
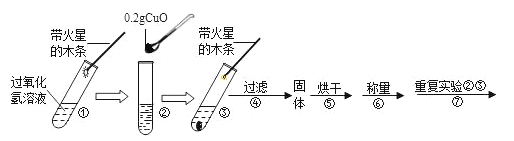
��1��������
��������� | ������ | ��������� | ���� |
_____�� �����ǵ�ľ����ȼ | ���ù��� _____g | ��Һ�������ݷų��� _____ | ������� �������� |
��2������ٵ�Ŀ����_____��
��3�����������ܱ�����ͭ���ֽ�ų������Ļ�ѧ��Ӧ�����ֱ���ʽΪ_____��