��Ŀ����
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ������������(��֪����������ʱ�ų�һ��������)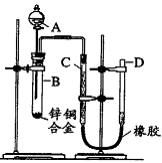
(1)ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���� ��
(2)ʵ����������У���������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú��ٽ��еIJ������У��ټ�¼C��Һ��λ�ã��ڴ�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ����ܼ�������ԡ�
�����ٽ��еIJ�����˳���� (�����)��
(3)��װ�õ������Եļ��鷽���Ǵ�Һ©����������D��ע�뾭���õ� ��ʹU������Һ����ƽ���رշ�Һ©�����������D�ܣ���˵��װ�õ������Ժã�
(4)��B�в����������������¼C��Һ��λ��ǰ���轫�¶Ȼָ������º� ��
(5)��ʵ����пͭ�Ͻ������Ϊag����ϡ�����ַ�Ӧ����������ΪV L��Ϊ����Ͻ���ͭ��������������ȱ�ٵ�һ�������� ��
| A����Ӧǰ����ϡ�������� | B����Ӧǰ����ϡ������������� |
| C��ʵ��ǰ��Ӧװ���п�������� | D��ʵ���������������ܶ� |
(7)ʵ������У���δ��ȴ�Ͷ�ȡ�������������п������������ (�� ��ƫ����ƫС������Ӱ�족)��
(8)��ָ����ʦ��Ϊ����������ʵ��װ�ã���������ܻ�ƫС��ԭ�������Ľ������� ��
(1)���DZ��������(2) �ܢ٢ۢڣ�3��ˮ���γ�Һ���һ��ʱ�䲻��ʧ����4������D��ʹ����Һ����ƽ����5��D
��6���⣺��п������Ϊx
H2����Ϊ0.08��0.09=0.0072g
Zn+H2SO4=ZnSO4+H2��
65 2
X 0.0072g
X=0.234g
Cu����������=(0.4-0.234)/0.4��100%=41.5%
���ԡ�����������Ҳ���֡�
��7��ƫ��
��8����Һ�����������Ƥ�ܽ�©�����Թ����ӵȺ����𰸡�
����

 ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������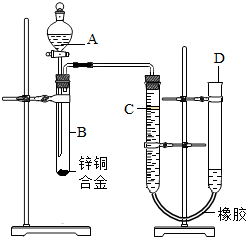 ��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������
��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������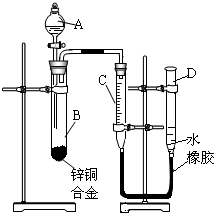 ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������ ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������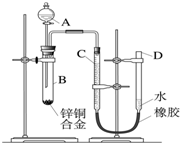 ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������