��Ŀ����
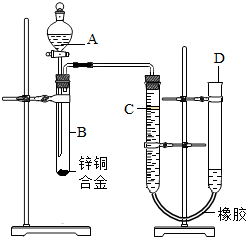 ��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������
��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ������������1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����
��ȥ�Ͻ���������Ĥ
��ȥ�Ͻ���������Ĥ
����2��ʵ����������У���������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú��ٽ��еIJ������У��ټ�¼C��Һ��λ�ã��ڴ�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ����ܼ�������ԣ�
�����ٽ��еIJ�����˳����
�ܢ٢ۢ�
�ܢ٢ۢ�
������ţ�����3����װ�õ������Եļ��鷽���Ǵ�Һ©����������D��ע�뾭���õ�
ˮ
ˮ
��ʹU������Һ����ƽ���رշ�Һ©�����������D�ܣ��۲쵽Һ�治���½�
�۲쵽Һ�治���½�
��˵��װ�õ������Ժã���4����B�в����������������¼C��Һ��λ��ǰ���轫�¶Ȼָ������º�
�رշ�Һ©���Ļ���
�رշ�Һ©���Ļ���
����5����ʵ����пͭ�Ͻ������Ϊag����ϡ�����ַ�Ӧ����������ΪV L��Ϊ����Ͻ���ͭ��������������ȱ�ٵ�һ��������
D
D
��A����Ӧǰ����ϡ�������� B����Ӧǰ����ϡ�������������
C��ʵ��ǰ��Ӧװ���п�������� D��ʵ���������������ܶ�
��6����aΪ0.4g��VΪ80mL����Ͻ���ͭ����������������ʵ�������£�H2���ܶ�Ϊ0.09g/L��
��7��ʵ������У���δ��ȴ�Ͷ�ȡ�������������п������������
ƫ��
ƫ��
���ƫ����ƫС������Ӱ�족������8����ָ����ʦ��Ϊ����������ʵ��װ�ã���������ܻ�ƫС��ԭ����
��һ�������������Թܺ͵�����
��һ�������������Թܺ͵�����
���Ľ����������Լ�ˮ�������Ϲ�ȥ
���Լ�ˮ�������Ϲ�ȥ
��������ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���ȥ�Ͻ���������Ĥ��Ҫ�����ſ�Һ���������ⶨ����������������������¼C��Һ�棬Ȼ��ʹ��Ӧ���У�����ַ�Ӧʱ�ڼ���C��λ�ã������Զ�ʣ�������д�����ע������ķ��������齺���ϼ��ϵ��ɼУ��������ܼ�����ˮ����������ܣ�һ��ʱ�������������Һ�治�½���˵�����������ã��ⶨͭп�Ͻ���п��ͭ��������������������п�����ᷴӦ����������ͭ�����������У�Ҫ������������������֪�����������������Ҫ֪���������ܶȣ�ͭп�Ͻ������Ϊag����aΪ0.4g��VΪ80mL��������������=0.08L��0.09g/L=0.0072g���Ӷ��������ͭ��������������������ʵ��װ�ã���������ܻ�ƫС��ԭ������һ�������������Թܺ͵����У��Ľ������ǣ����Լ�ˮ�������Ϲ�ȥ��
����⣺��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���ȥ�Ͻ���������Ĥ���ʴ�Ϊ����ȥ�Ͻ���������Ĥ
��2��Ҫ�����ſ�Һ���������ⶨ����������������������¼C��Һ�棬Ȼ��ʹ��Ӧ���У�����ַ�Ӧʱ�ڼ���C��λ�ã������Զ�ʣ�������д����������Ǣܢ٢ۢڣ��ʴ�Ϊ���ܢ٢ۢ�
��3�����齺���ϼ��ϵ��ɼУ��������ܼ�����ˮ����������ܣ�һ��ʱ�������������Һ�治�½���˵�����������ã��ʴ�Ϊ��ˮ���۲쵽Һ�治���½�
��4����B�в����������������¼C��Һ��λ��ǰ���轫�¶Ȼָ������ºرշ�Һ©���Ļ������ʴ�Ϊ���رշ�Һ©���Ļ���
��5��Ҫ������������������֪�����������������Ҫ֪���������ܶȣ��ʴ�Ϊ��D
��6��ͭп�Ͻ������Ϊag����aΪ0.4g��VΪ80mL��������������=0.08L��0.09g/L=0.0072g�����ݻ�ѧ����ʽ�ļ���������п��������0.234g���Ӷ��������ͭ����������=
��100%=41.5%���ʴ�Ϊ��41.5%
��7��ʵ������У���δ��ȴ�Ͷ�ȡ��������������ƫ�������п����������Ҳ��ƫ�ʴ�Ϊ��ƫ��
��8����������ʵ��װ�ã���������ܻ�ƫС��ԭ������һ�������������Թܺ͵����У��Ľ������ǣ����Լ�ˮ�������Ϲ�ȥ���ʴ�Ϊ����һ�������������Թܺ͵����У����Լ�ˮ�������Ϲ�ȥ
��2��Ҫ�����ſ�Һ���������ⶨ����������������������¼C��Һ�棬Ȼ��ʹ��Ӧ���У�����ַ�Ӧʱ�ڼ���C��λ�ã������Զ�ʣ�������д����������Ǣܢ٢ۢڣ��ʴ�Ϊ���ܢ٢ۢ�
��3�����齺���ϼ��ϵ��ɼУ��������ܼ�����ˮ����������ܣ�һ��ʱ�������������Һ�治�½���˵�����������ã��ʴ�Ϊ��ˮ���۲쵽Һ�治���½�
��4����B�в����������������¼C��Һ��λ��ǰ���轫�¶Ȼָ������ºرշ�Һ©���Ļ������ʴ�Ϊ���رշ�Һ©���Ļ���
��5��Ҫ������������������֪�����������������Ҫ֪���������ܶȣ��ʴ�Ϊ��D
��6��ͭп�Ͻ������Ϊag����aΪ0.4g��VΪ80mL��������������=0.08L��0.09g/L=0.0072g�����ݻ�ѧ����ʽ�ļ���������п��������0.234g���Ӷ��������ͭ����������=
| 0.4g-0.234g |
| 0.4g |
��7��ʵ������У���δ��ȴ�Ͷ�ȡ��������������ƫ�������п����������Ҳ��ƫ�ʴ�Ϊ��ƫ��
��8����������ʵ��װ�ã���������ܻ�ƫС��ԭ������һ�������������Թܺ͵����У��Ľ������ǣ����Լ�ˮ�������Ϲ�ȥ���ʴ�Ϊ����һ�������������Թܺ͵����У����Լ�ˮ�������Ϲ�ȥ
����������̽����ͭп�Ͻ���ͭ��п�����������IJⶨ����ɴ��⣬��������ͭ��п�����ʣ���������ṩ����Ϣ���У��й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ʵ�鷽��ʱ��Ҫע�������ٵ�ҩƷ����ķ��������ڶ�ʵ����Ʒ��������ۣ�Ҫ���������濼�ǣ�һ�Ƿ����Ƿ���У��ܷ�ﵽʵ��Ŀ�ģ�������Ƶķ������бȽϣ����ַ�������㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ