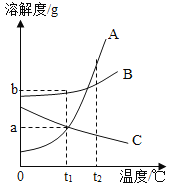
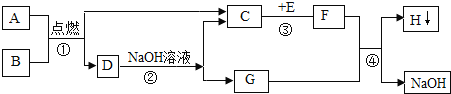
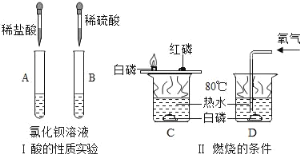
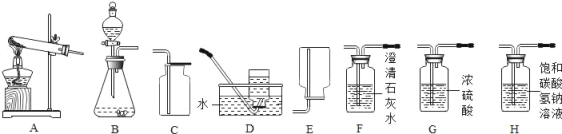
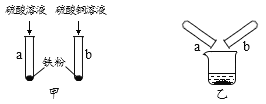
��Ŀ����
����Ŀ����ҵ�ϲ������ӽ���Ĥ���۵�ⱥ��ʳ��ˮ���ɵõ���Ũ�ȵ��ռ���Һ����NaOH 35%��48%����ij��ȤС������֤һ������������NaOH��Һ�Ƿ�ﵽ�˸�Ũ�ȱ������������²��������������㣺
��1����37%��Ũ��������200g7.3%�����ᣬ��Ҫ��ˮmL�����ܶ�Ϊ1gmL-1��������������һλС����____
��2���ɼ��û����������е�NaOH��Һ20g�������е��������Ƶ����ᣬ����Һ��pH=7ʱ����������100g���жϵ�����NaOH��Һ�Ƿ�ﵽ��Ũ�ȱ���____
���𰸡�160.5mL ������NaOH��Һ�ﵽ��Ũ�ȱ�
��������
��1������Ҫˮ�����Ϊx��
37%����200g-x��1gmL-1��=200g��7.3%
x��160.5mL�����160.5mL��
��2����������������Ƶ�Ũ��Ϊy��

![]() ��y=40%��40%��35%��48%֮�䣬�����Ǹ�Ũ�ȵ��ռ���Һ�����������NaOH��Һ�ﵽ��Ũ�ȱ���
��y=40%��40%��35%��48%֮�䣬�����Ǹ�Ũ�ȵ��ռ���Һ�����������NaOH��Һ�ﵽ��Ũ�ȱ���

��ϰ��ϵ�д�
�����Ŀ