��Ŀ����
ij��ѧʵ��С���ͬѧҪ�ⶨij���Ͻ�����Ԫ�����������������е������ɷ֣����μӷ�Ӧ���Ҳ�����ˮ��, ���ǵ�������:��7g���Ͻ���Ʒ���飬�μ�14.6%��ϡ���ᣬ�����ٲ�������ʱ��ǡ����ȥϡ����50g�������������Ϣ�ش��������⣻
��1�����Ͻ���Ʒ�ܽ�Ļ�ѧ����ʽΪ ��
��2�������Ʒ������������X���ı���ʽ ��
��3�������Ͻ���Ʒ�������������� ��
��4����������õĹ�Һ�������ˣ���ȥ��������ڷ�Ӧ����Һ�м���44.6gˮ�������������Һ��������������Ϊ ��
��5��������6300t�����Ͻ���Ҫ��������80%�ij�����ʯ������Ϊ ��
��1�����Ͻ���Ʒ�ܽ�Ļ�ѧ����ʽΪ ��
��2�������Ʒ������������X���ı���ʽ ��
��3�������Ͻ���Ʒ�������������� ��
��4����������õĹ�Һ�������ˣ���ȥ��������ڷ�Ӧ����Һ�м���44.6gˮ�������������Һ��������������Ϊ ��
��5��������6300t�����Ͻ���Ҫ��������80%�ij�����ʯ������Ϊ ��
��1��Fe+2HCl=FeCl2+H2������2��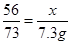 ����3��80%����4��12.7%����5��9000t��
����3��80%����4��12.7%����5��9000t��
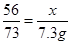 ����3��80%����4��12.7%����5��9000t��
����3��80%����4��12.7%����5��9000t���������������ϡ���ᷴӦ�����Ȼ�������������
�������Ʒ����������Ϊ
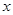 ��
��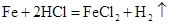
56 73
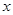 14.6%
14.6% 50g
50g
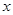 =5.6g
=5.6g��Ʒ�����������ı���ʽΪ
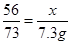
�����Ͻ���Ʒ��������������Ϊ

���ݷ�Ӧʽ�����ɵ��Ȼ���������Ϊ
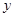 �����ɵ���������Ϊz������
�����ɵ���������Ϊz������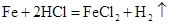
73 127 2
14.6%
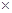 50g
50g  z
z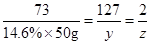
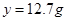 z=0.2g
z=0.2g������Һ������Ϊ:5.6g+50g+44.6g-0.2g=100g
���������Һ��������������Ϊ��
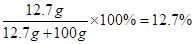
����Ҫ��������80%�ij�����ʯ������Ϊm��
�����ķ���ʽΪ
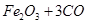

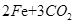
160 112
80%
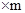
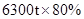
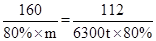
���m=9000t
��������ѧ����ʽ�ļ��������п��ص�Ҫ�����յ����ݣ�ÿ��ؿ������һ��ѹ������һ�������������ͣ�����Ҫ�������ܽᡣ

��ϰ��ϵ�д�
�����Ŀ

 ���ֱ��ʾ���������Ԫ�ص�ԭ�ӡ�����֪�⡢������ԭ�ӵ����ԭ�������ֱ�Ϊ��H-1 O-16��
���ֱ��ʾ���������Ԫ�ص�ԭ�ӡ�����֪�⡢������ԭ�ӵ����ԭ�������ֱ�Ϊ��H-1 O-16��