��Ŀ����
����Ŀ��С��Ҫ�ⶨijCu-Zn�Ͻ���ͭ��������������������ʵ�顣
��1����Ͳ��ȡ_______mL 98%Ũ���ᣨ�ܶ�Ϊ1.84g/mL�����100g 19.6%��ϡ���ᡣ
��2����������ɵ�ϡ����ȫ������ʢ��10g�Ͻ���Ʒ���ձ��У���Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
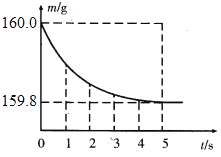
�ٲ�����������Ϊ______g��
�ڸúϽ���Ʒ��ͭ����������Ϊ_______��
�۷�Ӧ��������Һ������п��������������д�������̣���ȷ��0.1%��
���𰸡���1��10.9ml��2��0.2g 35% 6.1%
��������
�����������1����Һϡ�͵Ĺ��������ʵ��������䡣����ҪŨ���������Ϊx��
98%x =100g��19.6% ��x=20g ��
Ũ��������=20g��1.84g/mL=10.9ml
��2�����������غ㶨�ɻ�ѧ��Ӧǰ�����ʵ����������䡣�ʼ��ٵ�������Ϊ���ɵ�������������
��ͼ��֪����������������=160g-159.8g=0.2g ��
���û�ѧ����ʽ�����ݷ�Ӧ�������ȼ��ɼ�����Ͻ���п��������
��п������Ϊx ��������Һ������п������Ϊy��
Zn+H2SO4==ZnSO4+H2��
65 161 2
X y 0.2g
65��2=x��0.2g x=6.5g
�Ͻ���ͭ������=10g-6.5g=3.5g
�úϽ���Ʒ��ͭ����������=3.5g��10g ��100% =35% ��
161��2=y��0.2g x=16.1g
��Ӧ��������Һ������=6.5g+100g-0.2g=106.3g
��Һ������п����������=6.5g��106.3g ��100% =6.1%

����Ŀ�����泣ʳ�в�Ѫ������������ù�Ч�������װ�г�ʹ��һ�ִ�װ��������Ʒ��Ϊ��504˫�����������ǩ��ͼ��ʾ��ij��ѧ��ȤС���һ�����õġ�504˫������������Ʒ����Ũ�����Ȥ�����ʵ�����̽����
��������⡿���ù���ijɷ���ʲô��
���������ϡ��������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
��̼������Һ������������Һ��Ӧ������̼��Ƴ�������Ӧ�ķ���ʽΪ��
Ca(OH)2+Na2CO3==CaCO3��+2NaOH��
���������롿���ù����п��ܺ���Fe��Fe2O3��CaO��Ca��OH��2��CaCO3
��ʵ��̽����
ʵ����� | ʵ������ | ʵ����� |
��1��ȡ������������Թ��У�����������ˮ�ܽ⣬���ú�ȡ�ϲ���Һ�μ���ɫ��̪��Һ | �����ܽ�ʱ�Թ���ڷ��̣��Թܵײ��в������Һ��졣 | ������һ������ �� ���������ơ� |
��2����ȡ������������Թ��У��μ�������ϡ���ᡣ | ��������ʧ���д��� �� �������õ�dz��ɫ��Һ�� | ������һ������ �� �� һ������Fe2O3 |
��ʵ�����ɡ�
��1����ͬѧ��Ϊ��ͬѧ��ʵ���в��ܵó�һ����Ca(OH)2�Ľ��ۣ������� ��
��2����ͬѧ��ʵ�����������Fe2O3���������ᷢ����Ӧ��Fe2O3�����ᷢ����Ӧ�ķ���ʽΪ �� ����ͬѧ˼������Ϊ��ͬѧ��ʵ�鲢���ܵó�һ������Fe2O3�Ľ��ۣ������� �� ��
�����������ᷴӦ�����Ȼ����������Ȼ�����Ӧ�����Ȼ���������ҺҲ��dz��ɫ
�����������ʵ�鷽��������֤��

��ͬѧ������B����̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ�����������Թ��У��μ�������ϡ���ᣬ��������ͨ�����ʯ��ˮ | ��������ʧ��������ð���� ��Һ��Ϊ��ɫ��Һ��Ϊ��ɫ�� �����ʯ��ˮ����� �����ʯ��ˮ����� | ������һ������ �� ��Fe2O3 |
����˼�����ۡ�
��1����ͬѧ����ʵ������������C������Ϊ1.0 g��������ҺA�к��������Ƶ���������д��������̣�
��2����ͬѧ����ʵ���ô�����������������к������ʵ�������Ϊ1.6 g������B��CaCO3������Ϊ1.0 g���ۺ�����ʵ�鼰�ҡ���ͬѧ�����ݣ����ù���ijɷ��� ��
��3����Ϲ���ijɷ֣�����Ϊ�����504˫�����������������տ����е�ˮ���⣬�������տ����е� �Ӷ��ﵽ���������á�