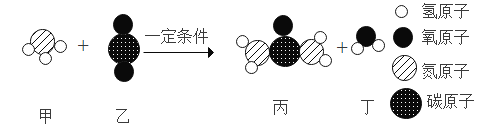
��Ŀ����
����Ŀ������˵����ȷ���ǣ� ��
A.3g̼��100g�����г��ȼ������103g������̼
B.![]() 98%��Ũ������
98%��Ũ������![]() ˮϡ�ͣ��ɵõ�������������Ϊ49%������
ˮϡ�ͣ��ɵõ�������������Ϊ49%������
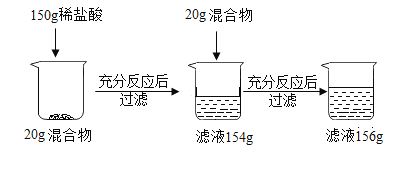
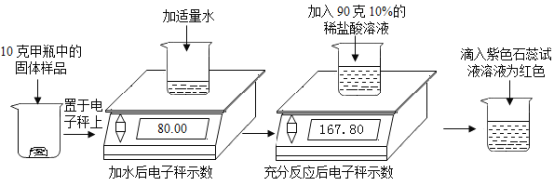
C.�ú����ʣ����ʲ����ᷴӦ��Ҳ������ˮ������10g��50gϡ������ȫ��Ӧ����ȥ���ʣ�����Һ������Ϊ55.4g�������ʵ�����������������Ϊ56%
D.![]() ��
��![]() �Ļ������98g10%��ϡ����ǡ����ȫ��Ӧ����ԭ���������Ԫ�ص�������1.6g
�Ļ������98g10%��ϡ����ǡ����ȫ��Ӧ����ԭ���������Ԫ�ص�������1.6g
���𰸡�CD
��������
A��̼��������Ӧ���ɶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��C+O2![]() CO2���μӷ�Ӧ��̼��������������12����16��2��=12��32����3g̼��8g����ǡ����ȫ��Ӧ������11g������̼����ѡ��˵������
CO2���μӷ�Ӧ��̼��������������12����16��2��=12��32����3g̼��8g����ǡ����ȫ��Ӧ������11g������̼����ѡ��˵������
B����Һϡ��ǰ�����ʵ��������䣬ˮ���ܶ�Ϊ1g/mL��100mLˮ������Ϊ100g����Ũ������ܶȴ���1g/mL��100mLŨ�������������100g����ϡ�ͺ����ʵ���������Ӧ����49%����ѡ��˵������
C����μӷ�Ӧ��������Ϊx
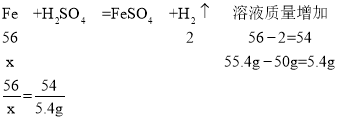
x=5.6g
����Ʒ�����ʵ���������=![]() ��100%=56%����ѡ��˵����ȷ��
��100%=56%����ѡ��˵����ȷ��
D��MgO��CuO��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��MgO+H2SO4�TMgSO4+H2O��CuO+H2SO4�TCuSO4+H2O������MgO��CuO�Ļ������98g10%��ϡ����ǡ����ȫ��Ӧ��������ѧ����ʽ����֪�����������Ԫ��û�з����ı䣬��Ȼ��������У���MgO��CuO�е���Ԫ����ת��Ϊˮ�е���Ԫ�أ������������Ԫ�ص�������ˮ����Ԫ�ص�������ȣ��ɷ���ʽ�ɵ�������ˮ����Ԫ�صĹ�ϵʽΪH2SO4��H2O��O
����ˮ����Ԫ��������x����
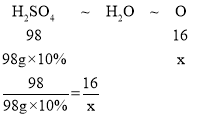
��ã�x=1.6g����ѡ��˵����ȷ��
��ѡ��CD��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ����֪ij���ڲ�ͬ�¶��µ��ܽ�����±���ʾ������������������Ϊ22%�ĸ�����Һ��50������ȴ����ʼ����������¶ȷ�Χ��_____��
�¶�/�� | 0 | 10 | 20 | 30 | 40 |
�ܽ��/g/100gˮ | 11.5 | 15.1 | 19.4 | 24.4 | 37.6 |