��Ŀ����
��8�֣����������װ�ûش��й����⣺
��1��д��ͼ�б��Т١��ڵ��������ƣ���Ϊ ����Ϊ ��
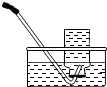
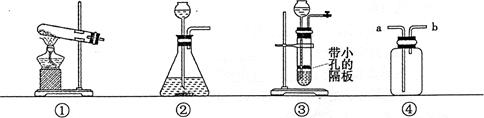
��2����������װ����ȡ������̼����A�з�����Ӧ�Ļ�ѧ����ʽΪ ��Ҫ��ȡ���ռ�һƿ����Ķ�����̼���壬ѡ�õ�װ���� ������ĸ���ţ�����װ�ýӿڵ�����˳���ǣ��ýӿ���ĸ˳������������ӣ� ��
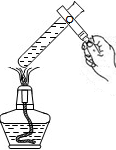
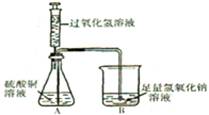
��3�������������巢��װ����ȡ��������A�з�����Ӧ�Ļ�ѧ����ʽΪ ����Ҫ����100g������������Ϊ6%��˫��ˮ��Һ����Ҫ30%��˫��ˮ��Һ g����Ҫ����ˮ�����Ϊ mL��

��1��д��ͼ�б��Т١��ڵ��������ƣ���Ϊ ����Ϊ ��
��2����������װ����ȡ������̼����A�з�����Ӧ�Ļ�ѧ����ʽΪ ��Ҫ��ȡ���ռ�һƿ����Ķ�����̼���壬ѡ�õ�װ���� ������ĸ���ţ�����װ�ýӿڵ�����˳���ǣ��ýӿ���ĸ˳������������ӣ� ��
��3�������������巢��װ����ȡ��������A�з�����Ӧ�Ļ�ѧ����ʽΪ ����Ҫ����100g������������Ϊ6%��˫��ˮ��Һ����Ҫ30%��˫��ˮ��Һ g����Ҫ����ˮ�����Ϊ mL��
��1����ƿ ˮ�� ��2�� CaCO3+2HCl==CaCl2+H2O+CO2�� ABE acbg
��3�� 2H2O2MnO2 2H2O+ O2�� 20 80
��3�� 2H2O2MnO2 2H2O+ O2�� 20 80
�����������1�� ����������ʶ��
��2�� ����ķ���װ��ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ����������������װ����ȡ������̼��˵�����ô���ʯ��ϡ���ᷴӦ����A�з�����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl==CaCl2+H2O+CO2����Ҫ��ȡ���ռ�һƿ����Ķ�����̼���壬����Ҫ�����������ռ�װ��ѡ�����ݣ�������ܶȺ��ܽ��ԣ����ڶ�����̼������ˮ���ܶȱȿ���������ֻ������ˮ���ռ�������ѡ�õ�װ����ABE�������ʱ��Ӧ��ȡ���ܽ����̹ܳ���������װ�ýӿڵ�����˳����acbg
��3���������巢��װ�����ʺϹ�����Һ���ڳ����·�Ӧ������A����ȡ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2MnO2 2H2O+ O2������Һ��ϡ�����У����ʵ��������䣬���Կ�����Ҫ30%��˫��ˮ��Һ������Ϊx������ʽΪ��100g��6%=x��30%��x=20g������Ҫ��ˮ������=100g-20g=80g�������ˮ�����Ϊ80 mL

��ϰ��ϵ�д�
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ