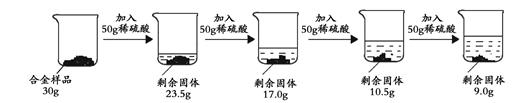
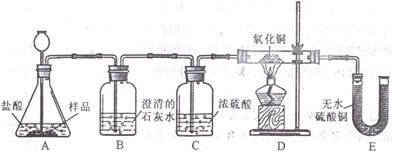
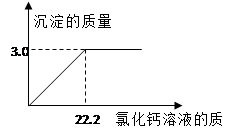��Ŀ����
��6�֣�����һƿ���õı�����������Ϊ10%��NaOH��Һ��Ʒ��Ϊ̽������ʣ�NaOH�Ϳ����е�CO2��Ӧ����Na2CO3����������⣬���á��ι��������ͼ��ʾ��װ�ý���ʵ�顣
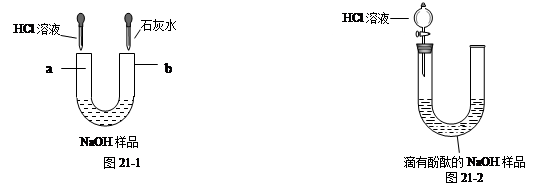
��1����ͼ46-1��ʾ��������Һ���ʣ��ֱ��ڡ��ιܵ����˵���HCl��Һ�ͳ����ʯ��ˮʱ���۲쵽������a�� ��b�� ��
��2����ͼ46-2��ʾ��ȡ��NaOH��Ʒ��Һ20g�ڡ��ι��У��������м��������ķ�̪��Һ��ͨ����Һ©������ι��е���HCl��Һ��������20gHCl��Һʱ��ǡ�÷�Ӧ��ȫ��
�ٵ���ϡ��������У����ι��е���Һ��ɫ�仯Ϊ ��
��ǡ����ȫ��Ӧʱ����������0.22g����ͨ������ó�20gNaOH��Ʒ��Һ������Na2CO3��������

��1����ͼ46-1��ʾ��������Һ���ʣ��ֱ��ڡ��ιܵ����˵���HCl��Һ�ͳ����ʯ��ˮʱ���۲쵽������a�� ��b�� ��
��2����ͼ46-2��ʾ��ȡ��NaOH��Ʒ��Һ20g�ڡ��ι��У��������м��������ķ�̪��Һ��ͨ����Һ©������ι��е���HCl��Һ��������20gHCl��Һʱ��ǡ�÷�Ӧ��ȫ��
�ٵ���ϡ��������У����ι��е���Һ��ɫ�仯Ϊ ��
��ǡ����ȫ��Ӧʱ����������0.22g����ͨ������ó�20gNaOH��Ʒ��Һ������Na2CO3��������
��6�֣�����ɫ�������� �� �а�ɫ�������� ����Һ�ɺ�ɫ��Ϊ��ɫ��
����Ʒ�к���Na2CO3������Ϊx
Na2CO3 + 2 HCl == 2NaCl+ H2O +CO2�� ��1�֣�
106 44
x 0.22g
106��44 = x��0.22g ��1�֣�
x =0.53g ��1�֣�
����Ʒ������Na2CO3������Ϊ0.53g��
����Ʒ�к���Na2CO3������Ϊx
Na2CO3 + 2 HCl == 2NaCl+ H2O +CO2�� ��1�֣�
106 44
x 0.22g
106��44 = x��0.22g ��1�֣�
x =0.53g ��1�֣�
����Ʒ������Na2CO3������Ϊ0.53g��
��1������Һ���ʣ�������̼���ƣ�̼���ƻ�����ᷴӦ���ɶ�����̼���������������ɣ���ʯ��ˮ��Ӧ����̼��ư�ɫ������
��2����̪�����죬�����������Һ����ɫ�����Ӧûʱ��������Һ���ɺ�ɫ��Ϊ��ɫ��
����Ʒ�к���Na2CO3������Ϊx
Na2CO3 + 2 HCl == 2NaCl+ H2O +CO2�� ��1�֣�
106 44
x 0.22g
106��44 = x��0.22g ��1�֣�
x =0.53g ��1�֣�
����Ʒ������Na2CO3������Ϊ0.53g��
��2����̪�����죬�����������Һ����ɫ�����Ӧûʱ��������Һ���ɺ�ɫ��Ϊ��ɫ��
����Ʒ�к���Na2CO3������Ϊx
Na2CO3 + 2 HCl == 2NaCl+ H2O +CO2�� ��1�֣�
106 44
x 0.22g
106��44 = x��0.22g ��1�֣�
x =0.53g ��1�֣�
����Ʒ������Na2CO3������Ϊ0.53g��

��ϰ��ϵ�д�
�����Ŀ