��Ŀ����
����Ŀ�����г�����һ����Ϊ�����ձ�ը�Ρ����²�Ʒ������˲��ȥ��ϴ�·����Գ�ȥ�Ķ���������ա�ijУ��ѧ��ȤС���ͬѧ������ʦ��ָ���¶Ըò�Ʒ��չ������̽�����

���Ķ���ǩ������Ʒ����_____��������
���������ϣ��١����ձ�ը�Ρ�����Ҫ�ɷ��ǹ�̼���ƣ�Na2CO4��������һ�ְ�ɫ�ᾧ������������ˮ��������ˮ��Ӧ������̼���κ����������
��������þ�Dz�����ˮ�İ�ɫ���塣
��������⣩�����ձ�ը�Ρ�����ˮ�γɵ���Һ������ʲô��
���������룩����һ��Na2CO3��H2O2
�������Na2CO3��NaOH
��������Na2CO3��H2O2��NaOH
��ʵ��̽����
��� | ʵ�鲽�� | ʵ������ | ʵ����� |
�� | ȡ���������ձ�ը�Ρ����ձ��У�������������ˮ��ֽ��� | ������ȫ�ܽ��γ���ɫ��Һ | |
�� | ȡ�����ٵ���Һ���Թ��У��������м��������������̷�ĩ���ٽ������ǵ�ľ�������Թܿ� | �д������ݲ�����ľ����ȼ | ����_____ |
�� | ȡ_____������ | �а�ɫ������ | ����̼���� |
�� | ȡ�����۵��ϲ���Һ�����У������еμ������Ȼ�þ��Һ���� | ��Һ��_____ | ������������ |
���ó����ۣ�����_____��ȷ��
��д����̼������ˮ��Ӧ�Ļ�ѧ����ʽ��_____��
��������չ��ͬѧ�ǽ�һ���ԡ����ձ�ը�Ρ��й�̼���Ƶĺ������вⶨ�����������������ͼ��ʾ��ʵ��װ�á�ͼ��װ��Bʢװ����������Һ��Cʢװ����ʯ��ˮ��DʢװŨ���ᣬE��F��װ�����ļ�ʯ�ң���Ҫ�ɷ֣������ƺ��������ƵĹ�����������ն�����̼��ˮ��������
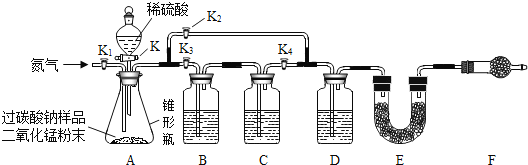
�ٳ���װ��E������������װ��װ�ã���������ԣ�װ��ҩƷ���۹رջ���K������K1��ͨ�뵪�����ܹرջ���K��K2������K1��K3��K4��ͨ��һ��ʱ��ĵ����������ιرջ���K1��K3��K4������K��K2��ֱ����Ӧ���ٽ��У����ٴγ���װ��E������������ʵ�鲽�����ȷ˳����_____��
A �٢ڢۢܢݢ�
B �ڢ٢ܢݢۢ�
C �٢ڢܢݢۢ�
D �ڢܢ٢ݢۢ�
���𰸡��� H2O2 CaCl2 �������ɣ������� һ Na2CO4+H2O= Na2CO3+H2O2 D
��������
[�Ķ���ǩ]��ͼ��֪�����ף�����ס�
[ʵ��̽��] ȡ�����ٵ���Һ���Թ��У��������м��������������̷�ĩ���ٽ������ǵ�ľ�������Թܿ��д������ݲ�����ľ����ȼ˵��������������Һ����H2O2��ȡCaCl2�������а�ɫ������˵������̼���ƣ�ȡ�����۵��ϲ���Һ�����У������еμ������Ȼ�þ��Һ�������۲����������ƣ�����Һ���������ɣ��������H2O2��CaCl2���������ɣ�������
[�ó�����]��Һ�к�H2O2��̼���ƣ������������ƣ��ʲ���һ��ȷ����̼������ˮ��Ӧ����̼���ƣ��������⣬��ѧ����ʽΪNa2CO4+H2O= Na2CO3+H2O2�����һ��Na2CO4+H2O= Na2CO3+H2O2��
[������չ] ����ʵ��Ҫ��, ��װ��װ�ã����������װ��ҩƷ���رջ���K��K2, ����K1��K3��K4, ͨ��һ��ʱ��ĵ���������װ��E�����������ιرջ���K1�� K3�� K4������K2��K���رջ���K������K1��ͨ�뵪�����ٴγ���װ��E����������ѡ��D��