题目内容
在实验考试前夕,小东同学在实验操作练习中,发现用完全相同的两份稀盐酸和相同质量的大块大理石、小块大理石分别反应,大块的反应速率要慢,原因是什么? _________ .
小东又发现盛放氢氧化钠的试剂瓶口有白色粉末.为了确定白色粉末的成分,他进行了如下的探究:
(1)小毛猜测白色粉末中含有碳酸钠,请指出他的理由: _________ (用化学方程式表示).
(2)为了确定自己的猜测,小东设计了如下的方案:取少量白色粉末于试管中,加水溶解配成溶液,再用 _________ 检验,看到溶液中有 _________ 产生,从而使自己的猜想得到了证实.
(3)玻璃中的SiO2可以和氢氧化钠发生类似的CO2的反应,则SiO2与氢氧化钠反应生成盐的化学式为 _________ .
(4)为了进一步确定白色粉末中碳酸钠的质量分数,小东取了2g白色粉末,加入足量的水充分溶解,再滴加澄清石灰水,充分反应后,过滤,得到了白色沉淀1g.
①在过滤时,需用到玻璃棒,它的作用是 _________ ;
②请你帮小东计算:白色粉末中碳酸钠的质量分数.
小东又发现盛放氢氧化钠的试剂瓶口有白色粉末.为了确定白色粉末的成分,他进行了如下的探究:
(1)小毛猜测白色粉末中含有碳酸钠,请指出他的理由: _________ (用化学方程式表示).
(2)为了确定自己的猜测,小东设计了如下的方案:取少量白色粉末于试管中,加水溶解配成溶液,再用 _________ 检验,看到溶液中有 _________ 产生,从而使自己的猜想得到了证实.
(3)玻璃中的SiO2可以和氢氧化钠发生类似的CO2的反应,则SiO2与氢氧化钠反应生成盐的化学式为 _________ .
(4)为了进一步确定白色粉末中碳酸钠的质量分数,小东取了2g白色粉末,加入足量的水充分溶解,再滴加澄清石灰水,充分反应后,过滤,得到了白色沉淀1g.
①在过滤时,需用到玻璃棒,它的作用是 _________ ;
②请你帮小东计算:白色粉末中碳酸钠的质量分数.
当稀盐酸的浓度相同时,反应物的接触面积越大,反应速率越快
(1)CO2+2NaOH=Na2CO3+H2O
(2)稀盐酸或澄清石灰水 气泡或有白色沉淀生成 (3)Na2SiO3
(4)①引流 ②53%
(1)CO2+2NaOH=Na2CO3+H2O
(2)稀盐酸或澄清石灰水 气泡或有白色沉淀生成 (3)Na2SiO3
(4)①引流 ②53%
试题分析:影响化学反应速率的因素有很多,比如:稀盐酸的浓度,反应温度,反应物的接触面积等,用完全相同的两份稀盐酸和相同质量的大块大理石、小块大理石分别反应,大块的反应速率要慢,原因是:当稀盐酸的浓度相同时,反应物的接触面积越大,反应速率越快
(1)氢氧化钠潮解后会与空气中的二氧化碳反应生成碳酸钠和水,反应化学方程式为:CO2+2NaOH=Na2CO3+H2O
(2)因为碳酸钠会与稀盐酸反应生成二氧化碳气体,因此可以加稀盐酸,看能否产生气泡或加石灰水看能否产生白色沉淀
(3)氢氧化钠能与二氧化碳发生化学反应,生成碳酸钠,SiO2和CO2的化学性质相似,所以SiO2与氢氧化钠反应生成硅酸钠,化学式为:Na2SiO3
(4)①过滤时玻璃棒的作用是引流,防止液体外溅,作用是:引流;
②根据化学反应:Na2CO3+Ca(OH)2=CaCO3↓+2NaOH,可知得到的白色沉淀1g即碳酸钙的质量,在根据反应中Na2CO3和CaCO3的质量关系,即可求出Na2CO3的质量,进一步可算出白色粉末中碳酸钠的质量分数
解:设白色粉末中碳酸钠的质量为X.
Na2CO3+Ca(OH)2=CaCO3↓+2NaOH
106 100
x 1g
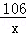 =
=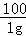 x=1.06g
x=1.06g因此
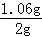 ×100%═53%
×100%═53%答:白色粉末中碳酸钠的质量分数为53%2CO3的化学性质,根据化学方程式计算

练习册系列答案
相关题目