��Ŀ����
��ѧʵ����ѧϰ��ѧ�Ļ��������������װ��ͼ�ش����⣮
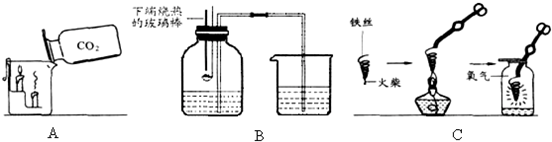
��1�������a��b������a
��2��д��Aͼ�в�������Ļ�ѧ����ʽ
��3��Bͼ�У��Ѷ�����̼���������������ȼ�ŵ�������ձ��У��²���������˵��������̼����
��4��Cͼ������״��˿��ĩ��ϵһ������������
��5��С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����
��6��С������˿��������ȼ��Ϊʲô������������̽�����±���������þ���Ͳ�ͬ��̼������˿��þ������˿ֱ����Ϊ0.4mm������������ȼ��ʱ��ʵ������ļ�¼����������������ش�
����Ϊ�������ǵ�ԭ���ǣ�

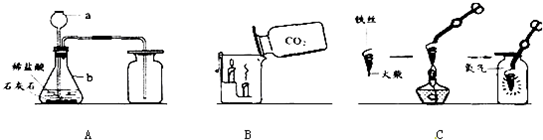
��1�������a��b������a
����©��
����©��
��b��ƿ
��ƿ
����2��д��Aͼ�в�������Ļ�ѧ����ʽ
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
����3��Bͼ�У��Ѷ�����̼���������������ȼ�ŵ�������ձ��У��²���������˵��������̼����
����ȼ
����ȼ
����֧��ȼ��
��֧��ȼ��
���ܶȱȿ�����
�ܶȱȿ�����
���ʣ���һ������ʵ�������е�Ӧ��Ϊ���
���
����4��Cͼ������״��˿��ĩ��ϵһ������������
��ȼ��˿
��ȼ��˿
����5��С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����
ȼ�ŵ���˿��������ƿ��
ȼ�ŵ���˿��������ƿ��
����6��С������˿��������ȼ��Ϊʲô������������̽�����±���������þ���Ͳ�ͬ��̼������˿��þ������˿ֱ����Ϊ0.4mm������������ȼ��ʱ��ʵ������ļ�¼����������������ش�
| ���� | þ�� | ��̼0.05%����˿ | ��̼0.2%����˿ | ��̼0.6%����˿ |
| ���� | ����ȼ�գ�����ҫ �۰⣬���� |
����ȼ�� ���ٻ��� |
����ȼ�� �������� |
����ȼ�գ��������� ����ȼ�գ��������� |
��˿�к��е�̼�����
��˿�к��е�̼�����
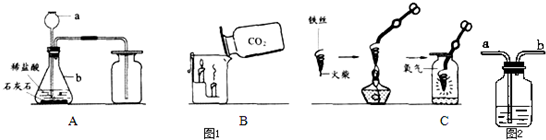
����������1�����ݳ������������ƽ��н��
��2�����������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��3������ʵ��������н��
��4������Cͼ������״��˿��ĩ��ϵһ��������������ȼ��˿���н��
��5������С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����ȼ�ŵ���˿��������ƿ�ڽ��н��
��6��������˿�к��е�̼����Ļ��ǽ��н��
��2�����������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��3������ʵ��������н��
��4������Cͼ������״��˿��ĩ��ϵһ��������������ȼ��˿���н��
��5������С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����ȼ�ŵ���˿��������ƿ�ڽ��н��
��6��������˿�к��е�̼����Ļ��ǽ��н��
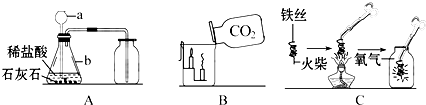
����⣺��1������a��b�����ƣ�����©������ƿ��
��2�������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽCaCO3+2HCl=CaCl2+CO2��+H2O��
��3���Ѷ�����̼���������������ȼ�ŵ�������ձ��У��²���������˵��������̼���в���ȼ����֧��ȼ�ա��ܶȱȿ�������һ������ʵ�������е�Ӧ��Ϊ���
��4��Cͼ������״��˿��ĩ��ϵһ��������������ȼ��˿��
��5��С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����ȼ�ŵ���˿��������ƿ�ڣ�
��6������������ش�
�������ǵ�ԭ���ǣ���˿�к��е�̼����ģ�
�ʴ�Ϊ����1������©������ƿ��
��2��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3������ȼ����֧��ȼ�ա��ܶȱȿ��������
��4����ȼ��˿��
��5��ȼ�ŵ���˿��������ƿ�ڣ�
��6������ȼ�գ��������䣻��˿�к��е�̼����ģ�
��2�������̼��Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽCaCO3+2HCl=CaCl2+CO2��+H2O��
��3���Ѷ�����̼���������������ȼ�ŵ�������ձ��У��²���������˵��������̼���в���ȼ����֧��ȼ�ա��ܶȱȿ�������һ������ʵ�������е�Ӧ��Ϊ���
��4��Cͼ������״��˿��ĩ��ϵһ��������������ȼ��˿��
��5��С����Cͼʵ��ʱ������ƿը���ˣ�����ܵ�ԭ����ȼ�ŵ���˿��������ƿ�ڣ�
��6������������ش�
| ���� | þ�� | ��̼0.05%����˿ | ��̼0.2%����˿ | ��̼0.6%����˿ |
| ���� | ����ȼ�գ�����ҫ �۰⣬���� |
����ȼ�� ���ٻ��� |
����ȼ�� �������� |
����ȼ�գ��������� |
�ʴ�Ϊ����1������©������ƿ��
��2��CaCO3+2HCl=CaCl2+CO2��+H2O��
��3������ȼ����֧��ȼ�ա��ܶȱȿ��������
��4����ȼ��˿��
��5��ȼ�ŵ���˿��������ƿ�ڣ�
��6������ȼ�գ��������䣻��˿�к��е�̼����ģ�
����������Ϊ�̲�ʵ������죬ּ�ڿ�������϶���ʾʵ�顢����ʵ�鼰ʵ��̽�����̵Ĺ۲졢�������ܽᡢ˼�������ۼ���˼��

��ϰ��ϵ�д�
�����Ŀ

 ��ѧʵ����ѧϰ��ѧ�ͽ��п�ѧ�о�����Ҫ������;����ѧ���������ʵ��Ʊ��������о����塢��������Ļ����������ͼ�ش��й����⣺
��ѧʵ����ѧϰ��ѧ�ͽ��п�ѧ�о�����Ҫ������;����ѧ���������ʵ��Ʊ��������о����塢��������Ļ����������ͼ�ش��й����⣺