��Ŀ����
����Ŀ����ѧ��ʦ����ijѧУ�о���ѧϰС��һ�����ⶨʵ������һƿ���õ�![]() �����Ƿ���ʣ���С���ͬѧ����������ʵ��̽����
�����Ƿ���ʣ���С���ͬѧ����������ʵ��̽����
������⣺����![]() ��û���ʣ�ȫ����
��û���ʣ�ȫ����![]() ��
��
����![]() �����ֱ��ʣ�________��
�����ֱ��ʣ�________��
����![]() ����ȫ���ʣ�ȫ����
����ȫ���ʣ�ȫ����![]() ��
��
�������ϣ�������Һ�ڳ����µ�![]() ���£�
���£�
����Һ |
|
|
|
| ���� | ���� | ���� |
��Ʒ���������ʵ�飺����������һ��ͬ��ɣ����ش��������⣮
ʵ�鲽�� | ʵ������ | ʵ����� |
�ٳ�ȡ�����������ƹ�����Ʒ | ������ɫ���� | ˵�����ù����У�һ������ ________���ѧʽ���� |
���ò�����պȡ�������г�ַ�Ӧ����ϲ���Һ����һС�� |
| ˵�����ù����У���һ������ ________ ���ѧʽ���� |
����������У������������ƹ�����Ʒ�õ��IJ�������������________���μӹ���![]() ��Һ��Ŀ����________
��Һ��Ŀ����________
ʵ����ۣ�ͨ��ʵ�飬˵������������________����ȷ�ģ�
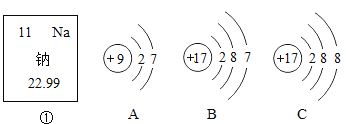
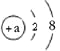
��չ����С��ͬѧΪ�ⶨ����![]() ����ı��ʳ̶ȣ�������������ɫ�������ˡ�ϴ��������Ƶ�������Ϊ
����ı��ʳ̶ȣ�������������ɫ�������ˡ�ϴ��������Ƶ�������Ϊ![]() ����ԭ��Ʒ��
����ԭ��Ʒ��![]() ����������Ϊ________ ������һλС������
����������Ϊ________ ������һλС������
��˼�����õ��������Ʊ��ʵ�ԭ����________���û�ѧ����ʽ��ʾ����
���𰸡�![]() ��
��![]() ��
��![]()
![]() �ձ���̼����ȫ���μӷ�Ӧ����
�ձ���̼����ȫ���μӷ�Ӧ����![]()
![]()
![]()
��������
�������������տ����еĶ�����̼���ʣ����ܲ��ֱ��ʣ�Ҳ����ȫ�����ʡ��ڼ������������Ƿ����ʱ���μ����������ݲ�������Ҫ֤���Dz��ֱ��ʣ����ȼ�����̼���ƣ����Լ�������Ȼ�����Һ��ͨ��������ɫ������֤����ͨ����������Ȼ�����Һ�ڳ�ȥ̼����֮�������Һ�Լ��ԣ�˵�������������ƣ��������ɳ�����������Ϸ�Ӧ�Ļ�ѧ����ʽ�������������������Ƶ����������н����
���������ֱ��ʣ��Ⱥ���NaOH�ֺ���Na2CO3��ʵ�����������Һ�еμ��Ȼ�����Һ����������ַ�Ӧ������������ɫ������˵�����ù����У�һ������Na2CO3�������ɳ�����ʣ����Һ��pH=11����Һ�Լ��ԣ�˵�����ù����У���һ������NaOH���������ƹ����׳��⣬��ǿ�ҵĸ�ʴ�ԣ���Ӧ�����ձ��г������μӹ���BaCl2��Һ��Ŀ������̼����ȫ���μӷ�Ӧ��ʵ����ۣ�ͨ��ʵ���֪��ԭ�����мȺ���NaOH�ֺ���Na2CO3��˵�����������в���������ȷ����
��չ������Ʒ��̼���Ƶ�����Ϊx��
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197
x 4.0g
![]()
x=2.15g
��Ʒ���������Ƶ�����Ϊ10g-2.15g=7.85g����ԭ��Ʒ��NaOH����������Ϊ![]() =78.5������˼�����õ��������Ʊ��ʵ�ԭ����2NaOH+CO2=Na2CO3+H2O��
=78.5������˼�����õ��������Ʊ��ʵ�ԭ����2NaOH+CO2=Na2CO3+H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±��г���������ڲ�ͬ�¶�ʱ���ܽ�ȣ�
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
�ܽ��/g | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
ij��ȤС����������ʵ�飺

��1��������Һ�е��ܼ���________������ʵ������еõ�����Һһ�����ڲ�������Һ����________����������ţ���������Ӧ��ʵ��������________��
��2��ʵ������У����ʵ���������һ����ȵ���________����������ţ���
��3��Ϊ�˷�ֹˮ����Ⱦ��С��ͬѧ��������·�������ʵ���ķ�Һ�� ����I�����ݼ�����ȴ��0�棬���ˣ����ɻ��յõ�KNO3����________g��
����II������Һ�м���________gˮϡ�ͣ��õ����ʵ���������Ϊ1%��KNO3ϡ��Һ��������Һ��Ϊ����ʹ�ã�
����Ŀ��С��ͬѧ�Թ��ƿ��������Ȥ���ڲ�ͬ��ʵ�������Ų�ͬ�����ã����½�����ȷ����
ʵ�� װ�� |
|
|
|
|
���� | �����ſ������ռ�������װ�� | ���ڸ��������̼��װ�� | ���ڲ������������װ�� | ����ҽԺ�����˹�������װ�� |
ѡ�� |
|
|
|
|
A. ![]() B.
B. ![]() C.
C. ![]() D.
D. ![]()