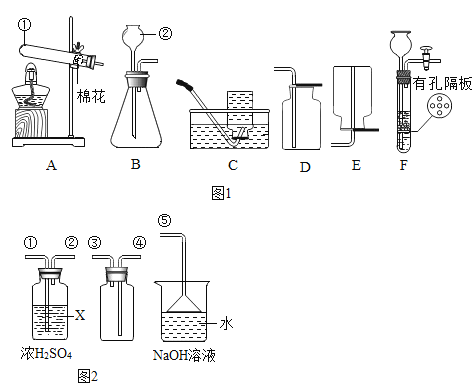
��Ŀ����
����Ŀ����ͼ��a��b��c�������ʵ��ܽ�����ߡ�����ͼ�ش�
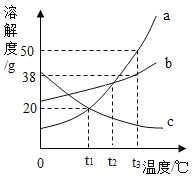
��1��_____��ʱ��a��c���ܽ����ȡ�
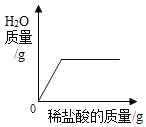
��2��t3��ʱ����20ga��b��c�������ʷֱ����ʢ��50gˮ���ձ��в��Ͻ��裬������Һ����������������С��ϵΪ_____����ѡ����ĸ����ͬ���������¶Ƚ��͵�t1��ʱ����Һ�����Ĵ�С��ϵΪ_____��
A c>a��bB b>a��cC a>b>cD b>a>c
��3����t2��ʱ����c���ʵIJ�������Һ��Ϊ������Һ�������й�˵������ȷ����_____������ĸ����
A �ܼ����������ܱ�СB ��Һ���������ܱ��
C ���ʵ���������һ�����D ���ʵ�����һ������
��4�����������Ȼ������Ƴ���Һ��20��ʱ����4��ʢ��200gˮ���ձ��У��ֱ����һ���������Ȼ��Ʋ�����ܽ⡣4��ʵ���������£�
ʵ����� | �� | �� | �� | �� |
�����Ȼ��Ƶ�������g | 9 | 36 | 81 | 90 |
��Һ������g | 209 | 236 | 272 | 272 |
�������еõ�����Һϡ�ͳ���������Ϊ0.9%��������ˮ�����ˮ��������_____g��
��������ʵ���������ȷ����_____������ţ���
a���٢�������Һ�Dz�������Һ
b���ۢ�������Һ�У����ʵ��������������
c��20��ʱ��200gˮ������ܽ��Ȼ��Ƶ�����Ϊ72g
d��20��ʱ�����ܼ�����������100gˮ�����ˣ��õ�����Ϊ36g�Ĺ���
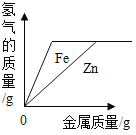
��5���������ֽ���������ɵĻ�����100gijϡ�����м���û������������ᷴӦ�ĵĽ�������ȫ����Ӧ��������������������������������ϵ��ͼ��ʾ��
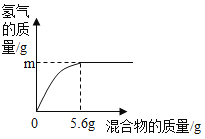
����˵����ȷ����_____������ţ���
a���������ΪZn��Al����m������0.2g
b���������ΪZn��Cu����mһ������0.2g
c���������ΪFe��Al����ϡ��������������������һ������7.3%
d���������ΪFe��Cu��mΪ0.1g����û������Fe����������һ����50%
���𰸡�t1 C B CD 791 ac ad
��������
��1����ͼ���Կ�������t1��ʱ��a��c���ܽ����ȣ�
��2��t3��ʱ���ܽ��a��b��c����20�� a��b��c�������ʷֱ���뵽ʢ��50��ˮ���ձ��в��Ͻ��裬aȫ���ܽ⣬b�ܽ����������c���ʹ�������Һ����������������С��ϵa��b��c�������¶Ƚ��͵�t1��ʱ����t3��ʱc��δ�ܽ�Ĺ���ȫ���ܽ⣬��ac�ܽ��һ���࣬b�ܽ����࣬��Һ�����Ĵ�С��ϵΪb��a=c��
��3��A�������ܼ�����ʱ���ʲ��䣬�ܼ����٣����������������ѡ��˵����ȷ��
B���������ʣ���ʱ������������ܼ����䣬���������������ѡ��˵����ȷ��
C�������¶ȣ��ܽ�ȼ�С�����Ա�ɱ�����Һ�����ʺ��ܼ�������û�䣬���������������䣬��ѡ��˵������
D���������ʣ���ʱ������������ܼ����䣬���������������ѡ��˵������
��4�����ݼ�ˮϡ��ǰ���������������֪0.9%��������ˮ�����ʵ�����Ϊ9g�������Һ����Ϊ![]() =1000g��������ˮ������Ϊ1000g-209g=791g��
=1000g��������ˮ������Ϊ1000g-209g=791g��
��������������Һ���ɢ�����Һ��֪200gˮ������ܽ�72gʳ�Σ���ˢ٢�Ϊ��������Һ���ۢ�Ϊ������Һ����a��ȷ��
�ۢܶ��Ǹ��¶��µı�����Һ�������������������ͬ����b����
����֪��20��ʱ��200gˮ������ܽ��Ȼ��Ƶ�����Ϊ72g����c��ȷ��
20��ʱ��������ҺΪ������Һ��ʣ��18gʳ��δ�ܽ⣬���ܼ�����������100gˮ��100gˮ������ܽ�ʳ��36g����˹��˵õ���������ӦΪ36g+18g=54g����d����
��5���������ȫ����������������Ϊ5.6 g������ݻ�ѧ��Ӧ�ķ���ʽ��Fe+2HCl=FeCl2+H2���ɼ������ʱ������������������0.2 g��ͬ���ɼ����5.6 gAl�����ᷴӦ������������������0.2 g��5.6gп�����ᷴӦ��������������С��0.2 g��ͭ�������Ӧ��
a���������ΪZn��Al��m���ܵ���0.2g����a��ȷ��
b���������ΪZn��Cu��mС��0.2g����b����
c���������ΪFe��Alʱ������ͬ������������ϡ����������������������������5.6 g��ʱ���������������Ϊ7.3 g�����Ի����ΪFe��Alʱ�������������������7.3 g����������������ʽ��֪��������������������һ������7.3%����c����
d���������ΪFe��Cu��mΪ0.1g������0.1g������Ҫ����������2.8g����������������������![]() ��100%=50%����d��ȷ��
��100%=50%����d��ȷ��