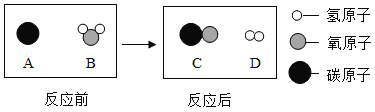
��Ŀ����
����Ŀ��ij̼�������Ȼ��ƵĻ���Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺
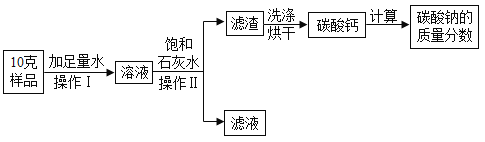
�ٲ������в�������������____���������������____��
��ʵ���еõ�0.05mol̼��ƣ������μӷ�Ӧ��̼���Ƶ�������____�ˣ�(����ݻ�ѧ����ʽ��ʽ����)��
��ʵ�������ijͬѧȡʵ���еõ�����Һ�������м���ϡ���ᣬ��Һ�����ı仯��ͼ��ʾ��
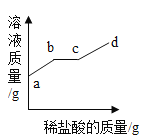
������ͼ��֪��Һ�е�������____��˵������ʵ��õ���̼���Ƶ���������____(����ƫ��������ƫС������ȷ��)����ԭ����_____��
���𰸡����裬�ӿ��ܽ��ٶ� ���� 5.3g NaCl��NaOH��Na2CO3 ƫС ����ʯ��ˮ�����㣬δ��̼������ȫ��Ӧ
��������
�ٲ������в������������ǽ��裬�ӿ��ܽ��ٶȣ������Ƿ��������Һ���һ�ֲ������������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������ʲ�����������ǹ��ˣ�
����̼���Ƶ����ʵ���Ϊx
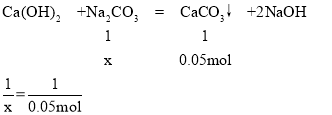
x=0.05mol
m=0.05mol��106g/mol=5.3g
����ͼʾ��֪����ʼ��Һ�������ӿ죬˵����Һ�������������ƣ����ּ�����˵�������̼���Ʒ�Ӧ�����������ɣ�������������������ʷ�����ͼ��֪��Һ�е�������NaCl��NaOH��Na2CO3��˵������ʵ��õ���̼���Ƶ���������ƫС����ԭ���DZ���ʯ��ˮ�����㣬δ��̼������ȫ��Ӧ��
�𰸣�
�ٽ��裬�ӿ��ܽ��ٶȣ����ˣ�
��5.3g
��NaCl��NaOH��Na2CO3��ƫС������ʯ��ˮ�����㣬δ��̼������ȫ��Ӧ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ұ���λͬѧ�ֱ�ȡ���ۺ�ͭ�۵ľ��Ȼ������ijϡ������ȫ��Ӧ�������������±���
�� | �� | �� | |
��������������(g) | 10 | 10 | 20 |
ϡ���������(g) | 100 | 120 | 100 |
��Ӧ�������ø�����������(g) | 4.4 | 4.4 | 14.4 |
����㣺
(1)_______������ϡ����ǡ����ȫ��Ӧ
(2)�����������������������Ϊ________��
(3)��������ϡ����ǡ����ȫ��Ӧʱ���������Һ�����ʵ�������������ȷ��0.1%��________��
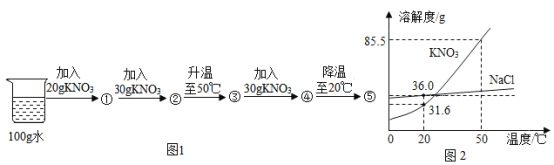
����Ŀ���ⶨijƷ�ƽ����������������ȡ20g��Ʒ�ƵĽ������Һ���ձ��У����ϵμ�25g������������Ϊ17.1%������������Һ����Ӧ�����е�������������Һ���������ձ�����ҺpH�仯�IJ�������������ʾ��
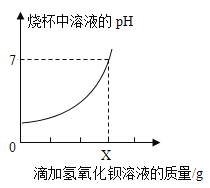
�μ�����������Һ������/g | 5 | 10 | X | 25 |
�ձ��в�������������/g | 1.165 | 2.33 | 4.66 | 4.66 |
��
�ٵ��ձ�����Һ��pH=7ʱ����������������Һ����X=______g��
�ڲ��20g��Ʒ�ƵĽ������Һ�������������______g��(���ݻ�ѧ����ʽ����)