��Ŀ����
����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
(1)Ϊ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա�ʵ��:
��� | ʵ��ҩƷ | �ֽ��¶�(��C) |
�� | 3.0gKC1O3��1.0gMnO2���Ȼ�ϼ��� | 150 |
�� | xg KC1O3�� 1. 0g CuO���Ȼ�ϼ��� | 170 |
���з�Ӧ�Ļ�ѧ����ʽ��_____________________________��
����x��ֵӦΪ_____________________________��
[ʵ�����]ʵ�� �������ֽ����������У���Ч����õ���_____________________________��
(2)��̽����Ӱ��˫��ˮ�ֽ����ʵ�ij�����أ�ʵ�����ݼ�¼����:
˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
�� | 50.0 g | 1% | 0.1 g | 9 mL |
�� | 50.0 g | 2% | 0.1 g | 16mL |
�� | 50.0 g | 4% | 0.1 g | 31mL |
��ʵ���У�����O2�����װ����__________________________________________(����)��
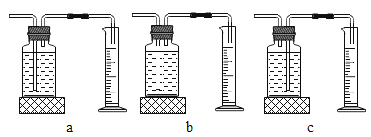
[ʵ�����]����ͬ�����£�_____________________________��˫��ˮ�ֽ��Խ�졣/span>
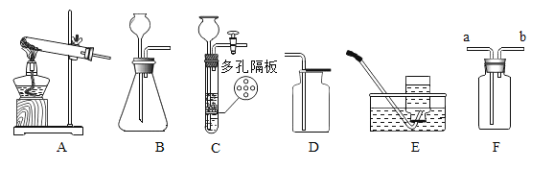
[��˼]������ͼװ�ý���ʵ�飺
ͨ���Ƚ�_____________________________Ҳ�ܴﵽʵ��Ŀ�ġ�
���𰸡�2KClO3![]() 2KCl + 3O2�� 3.0 �������� c ˫��ˮ��Ũ��Խ�� ��ͬʱ���ڣ�������ƽʾ���ı仯��С
2KCl + 3O2�� 3.0 �������� c ˫��ˮ��Ũ��Խ�� ��ͬʱ���ڣ�������ƽʾ���ı仯��С
��������
��1�����еķ�Ӧ��������ڶ��������������������·ֽ���Ȼ��غ���������Ӧ�Ļ�ѧ����ʽ��2KClO3![]() 2KCl + 3O2������������ص�������Ȳ��ܱȽϳ��������̺�����ͭ�Ĵ�Ч��������x��ֵӦΪ3.0��
2KCl + 3O2������������ص�������Ȳ��ܱȽϳ��������̺�����ͭ�Ĵ�Ч��������x��ֵӦΪ3.0��
ʵ����ۣ�
��Ӧ����Խ���Ч��Խ�ã�����ʵ���������ֽ����������У���Ч����õ��Ƕ������̣�
��2������̹ܽ����ܳ������ų��Լ�ƿ�е�ˮ����ѡc��
ʵ����ۣ�
�ӱ���ʵ�����ݿ�֪����ͬ�����£�˫��ˮ��Ũ��Խ��������������Խ�죻�����������Գ��ڵ�������ʣ�����ʵ��������ᣬ����������������ɵ��������������ʴ𰸣���ͬʱ���ڣ�������ƽʾ���ı仯��С��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�