��Ŀ����
����Ŀ����������ʵ�鳣��װ�ã��ش��й����⣺
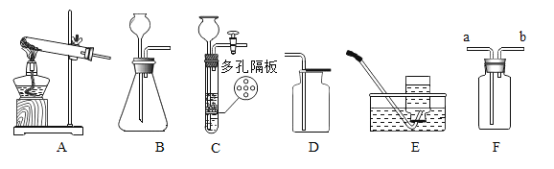
��1��д��װ��B��C����ͬ��������������______��ֻдһ�֣�
��2��ʵ�����ø��������ȡ������Ӧѡ�÷���װ��______������ĸ��ţ�����Ӧ�Ļ�ѧ���ֱ���ʽΪ______��
��3������Fװ���ռ��������������______�˽��루����a������b������д��ʵ�����ù���������ȡ�����Ļ�ѧ���ֱ���ʽ______��
��4��ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ���_________________��
���𰸡�����©���������� A �������![]() �����+��������+���� a ��������
�����+��������+���� a ��������![]() ˮ+���� ���Կ��Ʒ�Ӧ�ķ�����ֹͣ
ˮ+���� ���Կ��Ʒ�Ӧ�ķ�����ֹͣ
��������
��1��װ��B��C����ͬ�������������ƣ�����©���������ܣ�
��2��ʵ�����ø��������ȡ������Ӧѡ�ù������װ��A��������ؼ��Ȳ�������ء��������̺������Ļ�ѧ���ֱ���ʽΪ���������![]() �����+��������+������
�����+��������+������
��3����������ƿFװ���ռ��������������ܶȴ��ڿ�����������Ӧ�����ܽ����̹ܳ�����a�˽��롣����������������̻�ϲ���ˮ�������Ļ�ѧ���ֱ���ʽ����������![]() ˮ+������
ˮ+������
��4��ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ��ǣ�Cװ�ùر�ֹˮ�У�װ����ѹǿ�����ʹҩƷ���룬�Ӷ����Ʒ�Ӧ�ķ�����ֹͣ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
(1)Ϊ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա�ʵ��:
��� | ʵ��ҩƷ | �ֽ��¶�(��C) |
�� | 3.0gKC1O3��1.0gMnO2���Ȼ�ϼ��� | 150 |
�� | xg KC1O3�� 1. 0g CuO���Ȼ�ϼ��� | 170 |
���з�Ӧ�Ļ�ѧ����ʽ��_____________________________��
����x��ֵӦΪ_____________________________��
[ʵ�����]ʵ�� �������ֽ����������У���Ч����õ���_____________________________��
(2)��̽����Ӱ��˫��ˮ�ֽ����ʵ�ij�����أ�ʵ�����ݼ�¼����:
˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
�� | 50.0 g | 1% | 0.1 g | 9 mL |
�� | 50.0 g | 2% | 0.1 g | 16mL |
�� | 50.0 g | 4% | 0.1 g | 31mL |
��ʵ���У�����O2�����װ����__________________________________________(����)��
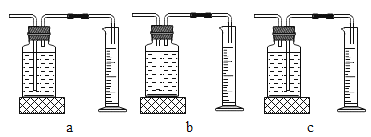
[ʵ�����]����ͬ�����£�_____________________________��˫��ˮ�ֽ��Խ�졣/span>
[��˼]������ͼװ�ý���ʵ�飺
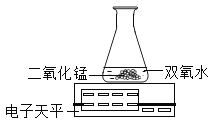
ͨ���Ƚ�_____________________________Ҳ�ܴﵽʵ��Ŀ�ġ�
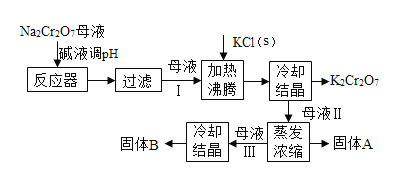
����Ŀ����ҵ�����ظ����ƣ�Na2Cr2O7���ᾧ���ĸҺ������������Fe3+�������ظ���أ�K2Cr2O7�������������̺�������ʵ��ܽ�������ܽ��������ͼ�����������з�Ӧǡ����ȫ���У���
���� | �ܽ�ȣ�g/100g H2O�� | ||
0�� | 40�� | 80�� | |
KCl | 28 | 40.1 | 51.3 |
NaCl | 35.7 | 36.4 | 38 |
K2Cr2O7 | 4.7 | 26.3 | 73 |
Na2Cr2O7 | 163 | 215 | 376 |
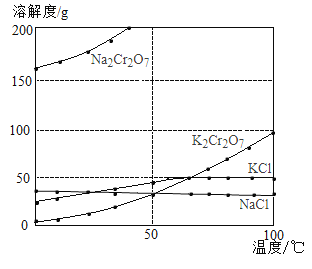
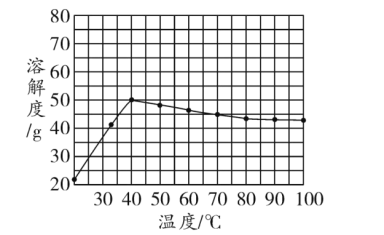
��1�����ܽ�����߿�֪��50��ʱ��K2Cr2O7��KCl��NaCl���������ܽ�ȵĴ�С��ϵΪ_____________��100��ʱ���õ�������K2Cr2O7��KCl��NaCl���ֹ������Ƴɱ�����Һ����Һ������С��ϵΪ_____________��
��2����40���������ʵı�����Һ������0������������������С����______________��
��3���Ӽ�Һ����pH��Ŀ����____________��
��4�������Ȼ��ع����Ӧ�Ļ�ѧ����ʽΪ___________�������ܽ�����ݿ�֪���÷�Ӧ�ܹ�������ԭ����____________��
��5��������Ҫ�õ�ĸҺ������A�IJ���Ϊ___________������Ũ��ԭ����___________��