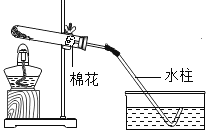
��Ŀ����
����Ŀ��ij�������д����� CuS ����������������ˮ�������ᷴӦ�����ʡ�ʵ������ �Ըÿ���Ϊԭ���Ʊ�CuSO4��5H2O���壬��������ȡ��ʽ̼��ͭ��
I. �Ʊ�CuSO4��5H2O����
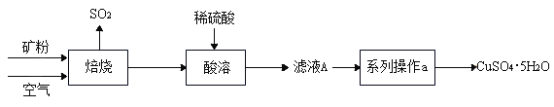
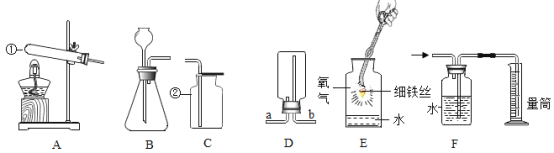
��1�������� CuS �ڿ����б��������� CuO����Ӧ����ʽΪ___��
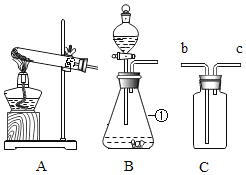
��2����ʵ�����У����� 98�����ܶ�1.84g/cm3��Ũ��������500 g��������10%��ϡ���ᣬ ��Ҫ����������Ͳ���ձ����������⣬����___��ϡ����___������������������ ��������
��3������ʵ���������ϵ�в��� a����Ҫ����Ϊ____�����½ᾧ��____��ϴ�������¸��������ѡ����ĸ��
II. �Ʊ���ʽ̼��ͭ
��4��ʵ�����Ʊ���ʽ̼��ͭ�ķ�Ӧԭ��Ϊ�����������ϵ����ʲ���������
![]() ____��+2Na2SO4��
____��+2Na2SO4��
��5���ֱ�ȡ 50mLһ��Ũ�ȵ�CuSO4��Һ��Na2CO3 ��Һ��ϣ����ַ�Ӧ���ձ��ײ����ִ�������ɫ�������������ϵ�֪����ɫ�����л���������ˮ�ļ�ʽ����ͭ[Cu2(OH)2SO4]��Ϊȷ�������ʣ������ˡ�ϴ����ȡ�����������Թ��У���������ϡ���Ὣ������ȫ�ܽ⣬�ٵμӼ���____��Һ���ְ�ɫ������֤������Cu2(OH)2SO4��
��6���������Ƚ���ʵ���Ʊ���ʽ̼��ͭ����Ӧ�����в���������£�
�¶�/�� | 60 | 65 | 75 | 80 | 90 |
������/g | 1.295 | 1.303 | 1.451 | 1.340 | 1.292 |
�ݴ˷����Ʊ���Ӧ���Ƶ������¶���____���Ʋ� 90��C������ٵ�ԭ����____��
��7���������Ͽ�֪��ʽ̼��ͭ������ж��֣�����xCu(OH)2yCuCO3��ʾ��ijС������ ����װ�òⶨij��ʽ̼��ͭ��Ʒ����ɣ������ϣ���ʽ̼��ͭ���ȷֽ�����ֳ������������ʯ�������������������ƵĻ���
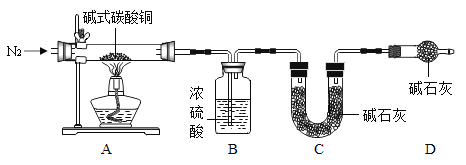
�ټ���ʱ����ͨ��N2��Ŀ����____��D װ�õ�������____��
�ڳ�ȡ 32.0g ��Ʒ����ַ�Ӧ��õ� 24.0g �����B ���� 3.6g��C ���� 4.4g������Ʒ�Ļ�ѧʽΪ____�����Ե� B �� C װ�õ�λ�ã�____�������������������������������Ʒ�Ļ�ѧʽ��
���𰸡�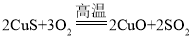 ��ͷ�ι� ���� A D CO2 �Ȼ��� 75�� �¶ȸ���75��ʱ����ʽ̼��ͭ�ֽ� ʹ��Ӧ����������ȫ���������װ�� ��ֹ�����еĶ�����̼��ˮ����U����� 2Cu(OH)2CuCO3 ��
��ͷ�ι� ���� A D CO2 �Ȼ��� 75�� �¶ȸ���75��ʱ����ʽ̼��ͭ�ֽ� ʹ��Ӧ����������ȫ���������װ�� ��ֹ�����еĶ�����̼��ˮ����U����� 2Cu(OH)2CuCO3 ��
��������
������ CuS �ڿ����б��������� CuO�Ͷ���������������Ӻͱ������������ᱵ����������ͨ�뵪������ʹ��Ӧ����������ȫ���������װ�á�
��1�����Ʊ�CuSO4��5H2O���������ͼ��֪�������� CuS �ڿ����б��������� CuO�Ͷ�������Ӧ�Ļ�ѧ����ʽΪ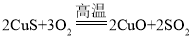 ��
��
��2����ʵ�����У����� 98�����ܶ�1.84g/cm3��Ũ�������� 500 g �������� 10%��ϡ���ᣬ ��Ҫ����������Ͳ���ձ����������⣬��ȡҺ��Ҫ�õ���ͷ�ιܣ��ʻ��н�ͷ�ιܡ�ϡ���̷��ȡ�
��3������ʵ���������ϵ�в��� a����Ҫ������ҺA��ȼ�ս��½ᾧ�����ˣ�ϴ�ӣ����¸������Ҫ����ΪA�����½ᾧ��D��ϴ�������¸��
��4���������غ㶨�ɿ�֪����Ӧǰ��ԭ�ӵ��������Ŀ�����䣬��Ӧǰ��2��ͭԭ�ӣ�2����ԭ�ӣ�4����ԭ�ӣ�15����ԭ�ӣ�2����ԭ�ӣ�2��̼ԭ�ӣ���Ӧ����2��ͭԭ�ӣ�2����ԭ�ӣ�4����ԭ�ӣ�2����ԭ�ӣ�1��̼ԭ�ӣ�13����ԭ�ӣ�������ϵ������Ƕ�����̼����![]() CO2��+2Na2SO4��
CO2��+2Na2SO4��
��5����ʽ����ͭ[Cu2(OH)2SO4]��������ϡ���Ὣ������ȫ�ܽ⣬��Һ�к�����������ӣ���������Ӻͱ������������ᱵ���������ٵμӼ����Ȼ�����Һ���ְ�ɫ������֤������Cu2(OH)2SO4��
��6���ɷ�Ӧ�����в�����ݿ�֪���¶���75��ʱ����������������Ʊ���Ӧ���Ƶ������¶���75����90��C������ٵ�ԭ�����¶ȸ���75��ʱ����ʽ̼��ͭ�ֽ⡣
��7���ٳ���ͨ�뵪������ʹ��Ӧ����������ȫ���������װ�ã��ʼ���ʱ����ͨ��N2��Ŀ����ʹ��Ӧ����������ȫ���������װ�ã���ʯ�ҿ������ն�����̼��ˮ����Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ����U����ܡ�
�ڳ�ȡ 32.0g ��Ʒ����ַ�Ӧ��õ� 24.0g ����������ɵ�����ͭ������Ϊ24.0g��Bװ������ˮ��B ���� 3.6g�������ɵ�ˮ������Ϊ3.6g����Ԫ�ص�����Ϊ![]() ��Cװ�����ն�����̼��C ���� 4.4g�������ɵĶ�����̼������Ϊ4.4g��̼Ԫ�ص�����Ϊ
��Cװ�����ն�����̼��C ���� 4.4g�������ɵĶ�����̼������Ϊ4.4g��̼Ԫ�ص�����Ϊ![]() ����ʽ̼��ͭ��̼Ԫ�غ���Ԫ�ص�������Ϊ
����ʽ̼��ͭ��̼Ԫ�غ���Ԫ�ص�������Ϊ![]() ��
��![]() ���ʸ���Ʒ�Ļ�ѧʽΪ2Cu(OH)2CuCO3��
���ʸ���Ʒ�Ļ�ѧʽΪ2Cu(OH)2CuCO3��
���Ե�B��Cװ�õ�λ�ã����ʯ�����ص�����Ϊ������̼��ˮ����������������3.6g+4.4g=8.0g����
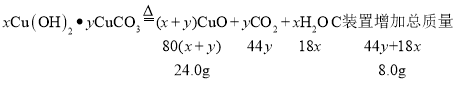
![]() x��y=2:1
x��y=2:1
����ȷ����ʽ̼��ͭ�Ļ�ѧʽ�������Ե� B �� C װ�õ�λ�ã��ܲ��������Ʒ�Ļ�ѧʽ��

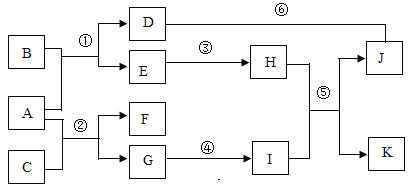
����Ŀ�����ܱ��������мס��ҡ��������������ʡ���һ�������·�Ӧ����÷�Ӧǰ����Ӧ�����е�t1��t2����ʱ�̸����ʵ��������±���ʾ������ a��b��c��d �ֱ��ʾ��Ӧ���ʵ��������������ݲ���ȷ���ǣ� ��
ʱ�� | �� | �� | �� | �� |
��Ӧǰ | 70g | 14g | 6g | 10g |
t1 ʱ�� | a | 11g | c | d |
t2ʱ�� | 42g | b | 40g | 10g |
A. a��56gB. b��8gC. c��17gD. d��10g