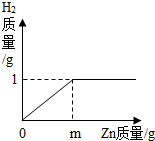
��Ŀ����
��1������ͬ�¶Ⱥ���ͬ����������£�����ѹǿ�����������Ŀ�����ȣ���ag̼��bg��������һ�ܱ������У��ṩһ������ʹ�����ڵ����ʷ�����ַ�Ӧ�����¶ȸ�ԭʱ����������ڵ�����ѹǿ�ڷ�Ӧǰ�䣮��
______
����������ܡ��ݣ���
��2����30��ʱ����ij�����ʵ���Һ136�ˣ��ֳ����ȷݣ�����һ�ݼ��������10�ˣ����ֻ�ܽ�2�˾Ͳ����ܽ��ˣ�����һ����ȴ��0��ʱ����������5.5�ˣ���ʱ��Һ��������������Ϊ20%�����������30��ʱ���ܽ��Ϊ______��
��3����������ƽ�������������и���һֻ�ձ���������ƽ�⣬���ձ��ֱ�ע���������������������ϡ���ᣬȻ������ֻ�ձ��зֱ������ͬ������þ��ͭ���Ͻ����ձ���������ȫ��Ӧ����ƽ�Ա���ƽ�⣬�Ͻ�������ͭ����������______��
| a |
| b |
| 3 |
| 8 |
��2����30��ʱ����ij�����ʵ���Һ136�ˣ��ֳ����ȷݣ�����һ�ݼ��������10�ˣ����ֻ�ܽ�2�˾Ͳ����ܽ��ˣ�����һ����ȴ��0��ʱ����������5.5�ˣ���ʱ��Һ��������������Ϊ20%�����������30��ʱ���ܽ��Ϊ______��
��3����������ƽ�������������и���һֻ�ձ���������ƽ�⣬���ձ��ֱ�ע���������������������ϡ���ᣬȻ������ֻ�ձ��зֱ������ͬ������þ��ͭ���Ͻ����ձ���������ȫ��Ӧ����ƽ�Ա���ƽ�⣬�Ͻ�������ͭ����������______��
��1���������⣬��Ӧǰ�������Ŀ���䣬��C+O2
CO2��2C+O2
2CO�����ж�̼�������г��ȼ�����ɶ�����̼��
����ag̼��bg����ǡ����ȫ��Ӧ����
C+O2
CO2
12 32
agbg
=
��
=
��������������ʣ�࣬��Ӧǰ�������ĿҲ���䣬��Ӧǰ��ѹǿҲ��ͬ����ˣ�������̼������������������
��
������
��
������ܣ�
��2����136����Һ�ֳ����ݣ���ÿ��68�ˣ�������һ����ȴ��0�棬����5.5g���ʣ�����Һ����68g-5.5g=62.5g��
��Һ�е�������62.5g��20%=12.5g��
��68����Һ��δ����֮ǰ����������Ϊ��12.5g+5.5g=18g���ܼ�����Ϊ��68g-18=50g��
��������10g��ֻ��2g�Ͳ��ܽ⣬˵���Ѵﱥ�ͣ���ʱ��Һ����������Ϊ��18g+2g=20g��
��30��ʱ����50��gˮ���ܽ�20g�����ʴﵽ���ͣ�
������30��ʱ���ܽ��ΪS��
=
��֮�ã�S=40g�����40g��
��3�����������������Ϊ2����Ҫþ������Ϊx����Ҫ��������Ϊy������
Mg+H2SO4�TMgSO4+H2��
24 2
x 2
=
x=24
2Al+3H2SO4�TAl2��SO4��3+3H2��
54 6
y 2
=
y=18
ͭ���Ͻ��������þ��������ȣ���ͭ������Ϊ24-18=6����������ͭ��������Ϊ18��6=3��1�����3��1��
| ||
| ||
����ag̼��bg����ǡ����ȫ��Ӧ����
C+O2
| ||
12 32
agbg
| 12 |
| ag |
| 32 |
| bg |
��
| a |
| b |
| 3 |
| 8 |
��������������ʣ�࣬��Ӧǰ�������ĿҲ���䣬��Ӧǰ��ѹǿҲ��ͬ����ˣ�������̼������������������
| a |
| b |
| 3 |
| 8 |
| a |
| b |
| 3 |
| 8 |
��2����136����Һ�ֳ����ݣ���ÿ��68�ˣ�������һ����ȴ��0�棬����5.5g���ʣ�����Һ����68g-5.5g=62.5g��
��Һ�е�������62.5g��20%=12.5g��
��68����Һ��δ����֮ǰ����������Ϊ��12.5g+5.5g=18g���ܼ�����Ϊ��68g-18=50g��
��������10g��ֻ��2g�Ͳ��ܽ⣬˵���Ѵﱥ�ͣ���ʱ��Һ����������Ϊ��18g+2g=20g��
��30��ʱ����50��gˮ���ܽ�20g�����ʴﵽ���ͣ�
������30��ʱ���ܽ��ΪS��
| 100g |
| S |
| 50g |
| 20g |
��֮�ã�S=40g�����40g��
��3�����������������Ϊ2����Ҫþ������Ϊx����Ҫ��������Ϊy������
Mg+H2SO4�TMgSO4+H2��
24 2
x 2
| 24 |
| x |
| 2 |
| 2 |
x=24
2Al+3H2SO4�TAl2��SO4��3+3H2��
54 6
y 2
| 54 |
| y |
| 6 |
| 2 |
y=18
ͭ���Ͻ��������þ��������ȣ���ͭ������Ϊ24-18=6����������ͭ��������Ϊ18��6=3��1�����3��1��

��ϰ��ϵ�д�
�����Ŀ