��Ŀ����
����Ŀ��ʵ����ϣ�ͬѧ�����ó���ʯ��ˮ��������еĶ�����̼��ʵ���з��ָ���ʯ��ˮ���dz̶ȴ��ڽϴ���죬�������ʵ��̽��Ӱ��ʯ��ˮ���dz̶ȵ����ء�
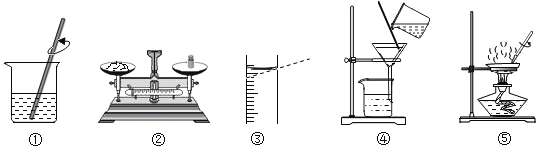
������ʵ�飩װ��ͼ������ʾ��

ʵ��1��ȡ���ͳ���ʯ��ˮ����ʵ�顣
ʵ����� | 1-1 | 1-2 | 1-3 | 1-4 |
���ͳ���ʯ��ˮ���/mL | 2.0 | 1.5 | 1.0 | 0.5 |
����ͨ��2 L������Ļ��dz̶� | ������ | ���� | �dz����� | ���� |
ʵ��2��������ʯ��ˮ����������ˮ������ʯ��ˮŨ�Ƚ���ʵ�飨��ҺŨ�ȱ仯�������Һ�ܶȱ仯���Բ��ƣ���
ʵ����� | 2-1 | 2-2 | 2-3 | 2-4 |
���ͳ���ʯ��ˮ���/mL | 2.0 | 1.5 | 1.0 | 0.5 |
��������ˮ���/mL | 0 | a | b | c |
����ͨ��3 L������Ļ��dz̶� | �dz����� | ���� | ������ | ������ |
����������ۣ�
��1��С�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ____________��
��2��ʵ��1��Ŀ��Ϊ____________��
��3��ʵ��2��a��b��c�������ݣ���ƺ�������________��
A 0.5 0.5 0.5 B 0.5 1.0 1.5
C 1.0 1.5 2.0 D 1.0 2.0 3.0
��4��ʵ��2�Ľ�����________��
����˼�����ۣ�
��5��ͨ���Ա�ʵ��1-1��2-1���ɵó�Ӱ��ʯ��ˮ���dz̶ȵ���һ������________��������________��
���𰸡�CO2 + Ca(OH)2 === CaCO3�� + H2O ̽��ʯ��ˮ����Ի��dz̶ȵ�Ӱ�졣 B ��ʯ��ˮ�����ͨ����������ͬ�������£�ʯ��ˮŨ��Խ���dz̶�Խ���ԡ� ͨ���������� ʵ��1-1��2-1�У�ʯ��ˮ�����Ũ����ͬ��ͨ������������ͬ�����dz̶Ȳ�ͬ��
��������
��1������ʯ��ˮ������еĶ�����̼��Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCO2 + Ca(OH)2 === CaCO3�� + H2O��
��2��ʵ��1�ĵı����dz���ʯ��ˮ�������Ŀ��Ϊ̽��ʯ��ˮ����Ի��dz̶ȵ�Ӱ�졣
��3��ʵ��2��̽������ʯ��ˮŨ�ȶԻ��dz̶ȵ�Ӱ�죬����Ӧ�ñ��ֱ��ͳ���ʯ��ˮ�����������ˮ�����ͬ����ѡB��
��4��ʵ��2�Ľ�������ʯ��ˮ�����ͨ����������ͬ�������£�ʯ��ˮŨ��Խ���dz̶�Խ���ԡ�
��5��ʵ��1-1��2-1�У�ʯ��ˮ�����Ũ����ͬ������ʵ��1ͨ����������Ϊ2L��ʵ��2ͨ���ͨ����������Ϊ3L���ɵó�Ӱ��ʯ��ˮ���dz̶ȵ���һ������ͨ������������������ʵ��1-1��2-1�У�ʯ��ˮ�����Ũ����ͬ��ͨ������������ͬ�����dz̶Ȳ�ͬ��

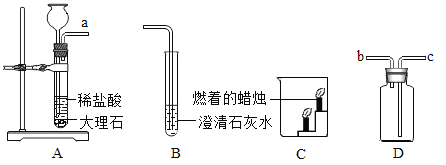
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�����Ŀ������ʵ����������ȷ����
ѡ�� | A | B | C | D |
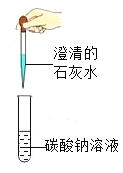
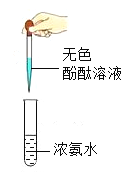
ʵ�� |
|
|
|
|
ʵ������ | ����ȼ�գ��������䣬�ų��������ȣ����ɺ�ɫ���� | ���ְ�ɫ���� | ��Һ����ɫ��Ϊ��ɫ | �������ݣ���Һ��Ϊdz��ɫ |
A.AB.BC.CD.D