��Ŀ����
�������ͼ��ʾʵ��װ�ûش����⣺
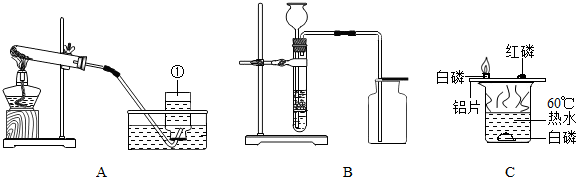
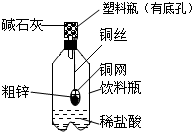
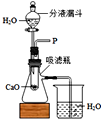
��1��ͼ�б�Ţٵ�������______��
��2��Aװ�ÿ�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ______���÷�����ȡ��������Ҫʵ�鲽���У��ټ��ȣ������Թ���װҩƷ���̶����ۼ��װ�������ԣ�������ˮ���ռ���������ֹͣ���ȣ������ܴ�ˮ����ȡ������ȷ��ʵ�����˳����______��������š�
��3��Bװ�ÿ�����ʵ������ȡ���ռ�������̼����Ӧ�Ļ�ѧ����ʽΪ��______��ʵ���ҿ�����Zn+H2SO4�TZnSO4+H2����ȡ������______����ܡ����ܡ�����Bװ����ȡ���ռ�������
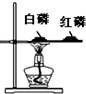
��4��Cʵ������̽��ȼ�յ�������ʵ��ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա�______������õ��ģ�

��1��ͼ�б�Ţٵ�������______��
��2��Aװ�ÿ�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ______���÷�����ȡ��������Ҫʵ�鲽���У��ټ��ȣ������Թ���װҩƷ���̶����ۼ��װ�������ԣ�������ˮ���ռ���������ֹͣ���ȣ������ܴ�ˮ����ȡ������ȷ��ʵ�����˳����______��������š�
��3��Bװ�ÿ�����ʵ������ȡ���ռ�������̼����Ӧ�Ļ�ѧ����ʽΪ��______��ʵ���ҿ�����Zn+H2SO4�TZnSO4+H2����ȡ������______����ܡ����ܡ�����Bװ����ȡ���ռ�������
��4��Cʵ������̽��ȼ�յ�������ʵ��ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա�______������õ��ģ�
��1����������Ϊ����ƿ��
��2��������طֽ�Ļ�ѧ����ʽ2KMnO4
K2MnO4+MnO2+O2��������ˮ���ռ�������Ҫ�Ȱѵ��ܴ�ˮ���г�����Ȼ��Ϩ��ƾ��ƣ���ȷ��ʵ�����˳���� �ۢڢ٢ܢޢݣ�
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�����������ܶ�С�ڿ������ʲ����������ſ������ռ���
��4��ʵ��Ҫ�ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա� ˮ�а�����Ƭ�ϰ�������õ��ģ�
�ʴ�Ϊ��
��1������ƿ��
��2��2KMnO4
K2MnO4+MnO2+O2�����ۢڢ٢ܢޢݣ�
��3��CaCO3+2HCl�TCaCl2+H2O+CO2���� ���ܣ�
��4��ˮ�а�����Ƭ�ϰ��ף�
��2��������طֽ�Ļ�ѧ����ʽ2KMnO4
| ||
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�����������ܶ�С�ڿ������ʲ����������ſ������ռ���
��4��ʵ��Ҫ�ó���ȼ��ȼ����Ҫ�������Ӵ��Ľ��ۣ���ͨ���Ա� ˮ�а�����Ƭ�ϰ�������õ��ģ�
�ʴ�Ϊ��
��1������ƿ��
��2��2KMnO4
| ||
��3��CaCO3+2HCl�TCaCl2+H2O+CO2���� ���ܣ�
��4��ˮ�а�����Ƭ�ϰ��ף�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ