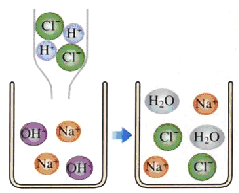
��Ŀ����
����Ŀ��У��ѧ��ȤС���������۵�ijЩ���������۽���̽����������A���ࡢB���ࡢC�����̱���Ħ��������Ҫ�ɷ֡�
A���� | B���� | C���� |
����������̼��� | ̼��� | ̼��� |
�������ϣ�������������������ᷴӦ�����κ�ˮ��Ҳ�ܺͼӦ�����κ�ˮ������������г���Ħ�������⣬�������ʾ������ᡢ�Ӧ����
ʵ��һ����֤ A�����к�������������̼��ƣ�ʵ��������£�

��1���������x�ľ������������_____��
��2���Ӳ���۵�̽�������֪A������һ������̼���Ħ������ԭ���û�ѧ����ʽ��ʾΪ_____��
��3��Ϊ��һ��֤�������������ڣ���������ó����м���_____�����۲쵽_____������֤�������������ڡ�
ʵ������Ƚ�B �����C������̼��ƺ����Ķ��٣�ʵ��������£�
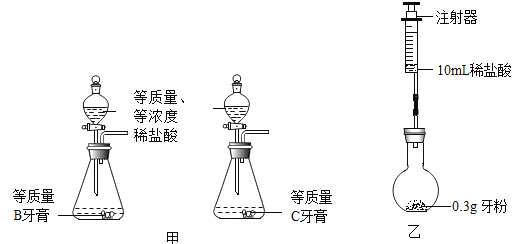
���� | ʵ����� | ʵ������ |
�� | ��ͬһ��װ���Ⱥ��������ʵ�飬װ��ҩƷ��ͼ�� | |
�� | ��Һ©������ע��һ����ϡ���ᣬȻ��رջ��� | �����ݲ��� |
�� | ���ڷ�Ӧ�������ٴ�Һ©��������ע��һ����ϡ���ᣬȻ��رջ��� | ���������� |
�� | ������Ӧ��װ�ü�ҩƷ�����������Ƚ� | ����B�����װ�ü�ҩƷ�����������ڼ���C���۵�װ�ü�ҩƷ�������� |
��4��ʵ�����Ϊ_____��
��5������۵�������_____��
��6����ͬѧ��Ϊ��ʵ��ǰ���Բ����װ�õ������ԣ����Ƿ���ͬ����˵��ԭ��_____��
ʵ�������ⶨC������̼��Ƶĺ���
��7������һ�������Ķ�ʵ�����װ�úͻ������裬Ҫ�ⶨ������̼��Ƶ�������������Ӧ�ⶨ��������_____��
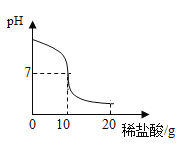
��8����������ȡC����0.3g����ͼ��װ�ã��ٽ�ע������10mLϡ����Ѹ��ѹ����ƿ�С�ʵ���¼���£�
ʱ��/min | 1 | 3 | 5 | 7 | 8 | 9 | 10 |
ע��������/mL | 40.0 | 59.0 | 60.0 | 60.4 | 60.5 | 60.5 | 60.5 |
�ۺϷ�������ʵ����̺����ݣ�������������������_____mL���������������������������������C������̼��Ƶĺ�����
���𰸡��ò�����պȡ������Һ��pH��ֽ�ϣ���ɫ�������ɫ���Ƚ� ![]() NaOH��Һ ���������ܽ� C������̼��Ƶĺ�������B������̼��Ƶĺ��� �ж������е�̼�������ȫ��Ӧ ��ͬ����Ӧ���ɵĶ�����̼��ͨ������ɢ�ݵ������У�װ���Ƿ�©����Ӱ��ʣ��������� ��ӦǰC���۵����� 50.5
NaOH��Һ ���������ܽ� C������̼��Ƶĺ�������B������̼��Ƶĺ��� �ж������е�̼�������ȫ��Ӧ ��ͬ����Ӧ���ɵĶ�����̼��ͨ������ɢ�ݵ������У�װ���Ƿ�©����Ӱ��ʣ��������� ��ӦǰC���۵����� 50.5
��������
��1����pH��ֽ�ⶨ��ҺpH�ķ����ǣ��ò�����պȡ������Һ�μ�pH��ֽ�ϣ���ɫ�������ɫ���Ƚϣ�������Һ��pH��
��2��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3����Ϊ���������ܺ��������Ʒ�Ӧ��̼��Ʋ����������Ʒ�Ӧ��������������ó����м�������������Һ�����۲쵽���������ܽ⣬����֤�������������ڣ�
��4�������������ࡢ������������������ϡ���ᷴӦ�����ݷ�Ӧ���������ļ���Ҳ�������ɶ�����̼�������������ٿ�ȷ�����ࡢ������̼��Ƶ��������٣�����ʵ�����Ϊ��C������̼��Ƶĺ�������B����̼��Ƶĺ�����
��5������۵������ǣ��ж������е�̼�������ȫ��Ӧ��
��6����ͬѧ��Ϊ��ʵ��ǰ����Ҫ�ⶨװ�õ������ԣ���Ϊ��ʵ�鲻��ֱ���ռ��ⶨ������̼��������ʵ��װ���Ƿ�©����Ӱ��ⶨ��������Ը�ʵ��ǰ����Ҫ�ⶨװ�õ������ԣ�
��7��Ҫ�ⶨ������̼��Ƶ�������������Ҫ�ⶨ������̼��Ƶ�����������ͨ��������̼���������̼��Ƶ���������˻�Ӧ�ⶨ�������з�ӦǰC���۵�������
��8��ע��������ʼ������10mL�����ն���Ϊ60.5mL��������50.5mL����������������������50.5mL��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д� ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�����Ŀ����20.0gϡ������μ��뵽10.0g������������Ϊ40%������������Һ�У��ߵμӱ߽��裬����ϡ����ĵμӣ���Һ��pH�仯�����ͼ��ʾ����Һ���¶ȱ仯�����ʾ�������Ƿ�Ӧ����������ɢʧ��
��Ӧʱ�䣨s�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
��Һ�¶ȣ��棩 | 20.5 | 20.6 | 20.7 | 20.8 | 20.9 | 20.8 | 20.7 |
��1����ϡ����������������Һǡ����ȫ��Ӧʱ����Һ���¶���_____��
��2������ϡ���������ʵ���������_________����ȷ��0.1%��
����Ŀ��ij��ѧС���ͬѧ����ʦ��ָ���£��������װ����֤̼��Ƶ����ʵ�ͬʱ��̽���������������̼�ڼ��ȵ��������Ƿ�Ҳ�ܷ�����Ӧ����̼��ơ�
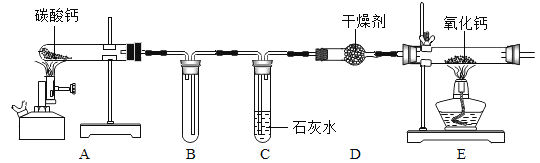
��1��C�еķ�Ӧ����ʽΪ_____��
��2��ʵ���С��ͬѧ��E�й���ijɷֽ���̽����
[���������]E�еĹ������Ϊa�����ƣ� b��������̼��ƣ� C ̼���
[����ʵ��]
ʵ�鲽�� | ���� | ���ۻ�ѧ����ʽ |
��ȡһ������E�й������Թ��У�������һ������ˮ���� | �а�ɫ������ | �ܷ�֤������c����_____�����ܡ���������_____�� |
��ȡһ������E�й������Թ��У�������һ������ˮ���μ�_____��Һ�� | �Թ���Һ���Ժ�ɫ | �Թ��з�Ӧ�Ļ�ѧ����ʽ_____�� |
�����������Թ��еμ�������ϡ���ᡣ | ��Һ�������ݲ��� | ֤����ȡһ����E�й���ijɷ���_____���ѧʽ���� |
��3�����պ�̼���50�˵�ʯ��ʯ����ȫ�ֽ�����������Ƶ����ʵ����Ƕ��٣� _____�����ݻ�ѧ����ʽ��ʽ���㣩