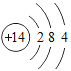
��Ŀ����
����Ŀ��2018��5��18���ҹ���һ�ҹ�����ĸ���Գɹ�����ĸ���������Ԫ��ʹ���˻�ͭ��Ϊ�ⶨij��ͭ������Ͻ��н���ͭ��п����ͭ��������������ȤС��ͬѧ��ȡ20g��ͭ��ĩ���ձ��У���80gϡ������Ĵμ��룬��ַ�Ӧ�����ʵ���������±���ʾ:
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
����ϡ�����������g�� | 20 | 20 | 20 | 20 |
�ձ���ʣ�����������g�� | 39.92 | 59.84 | 79.80 | 99.80 |
��1����ͭ��ĩ��ȫ��Ӧ����������������Ϊ__g��
��2��������ʵ�����Һ�е�������___��
��3���û�ͭ��ͭ����������Ϊ����__?��д ��������̣�
���𰸡�0.2 ���������п����H2SO4��ZnSO4�� 67.5%
��������
���������غ㶨�ɿ�֪�������������ļ�������Ϊ���������������Կ����������������������������������Ͷ�Ӧ�Ļ�ѧ����ʽ�����ͭ��ͭ������������
�⣺���������غ㶨�ɿɵã���������������Ϊ20g+80g-99.80g=0.2g������ÿ����20gϡ��������0.08g�������������������������0.04g,������������Ե�����ʵ�����Һ�е�������ʣ�����������ɵ�����п���� H2SO4��ZnSO4��
�裺��ͭ��ͭ������Ϊx��
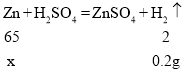
![]()
x=6.5g
��ͭ��ͭ������������![]()
�𣺸û�ͭ��ͭ����������Ϊ67.5%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
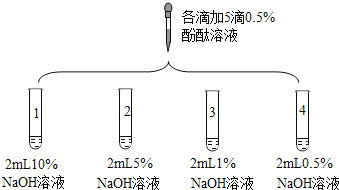
Сѧ��10����Ӧ����ϵ�д�����Ŀ��2018 ��Ϸ����п�����ʵ������У���Ҫ�� NaOH ��Һ�μӷ�̪��Һ�����������ϰʱ������ͬѧ�۲쵽�����벻���������е���Һ����ܿ��ʳ���ɫ���е���Һ�г��ְ�ɫ�����
��������⣩����ʵ���У��������벻���������ԭ����ʲô�أ�
���������ϣ���̪��Һ�ɷ�̪�������ھƾ����ƶ��ɡ�
����������裩������ɫ��ȥ�������Ƿ�̪�� O2 �����˷�Ӧ�йء�
������ɫ��ȥ�������� NaOH ��Һ�Ϳ����е� CO2 ��Ӧ�йء�
������ʵ�飩
ʵ�� | ʵ����� | ʵ������ | |
1 | �� NaOH ��Һ������У� ����ȴ�����Һ�� �����̪���������Ϸ�����һ��ֲ���� | ��Һ��죬��һ�����ɫ��ʧ | |
2 | ��ʢ�� 2 mL ����������Һ���Թ���ͨ������������̼���ٵ����̪��Һ | ��Һ��죬һ��ʱ���ɫ����ȥ | |
3 |
| 1���Թ� 0.1min ��ɫ��ȥ 2���Թ� 5min ��ɫ��ȥ 3���Թ� 30min ��ɫ���Ա�dz 4���Թ� 120min ��ɫ�����Ա仯 ��ע��min ��ʾ���ӣ� | |
4 | ȡ 3 ֧�Թܣ��ֱ���� 2 mL ˮ���ٷֱ���� 5 �β�ͬŨ�ȵķ�̪��Һ�� | ��̪��ҺŨ��/% | ���dz̶� |
5 | ���������� | ||
2 | ���������� | ||
0.5 | ������ | ||
����������ۣ�
��1��ʵ�� 1 �У��� NaOH ��Һ��в��μ�ֲ���͵�������_____��
��2����������������������_____���û�ѧ����ʽ��ʾ����
��3����ʵ�� 3 ���ֵ�����ó�������������_____��_____��
��4����ʵ�� 4 ���������֪��ͬ�¶ȡ�ѹǿ��ͬʱ����̪��ˮ�е��ܽ��_____��̪�ھƾ��е��ܽ�ȣ���������������С������������������