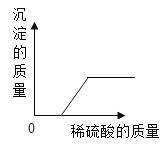
��Ŀ����
����Ŀ������ۡ��ۼ���ѧ������ϵ��һ���ǻ�ѧѧ�Ƶ��ص㣮
��1��A��B��C��D��ʾ�������ʣ�����ʾ��ͼ������ʾ��
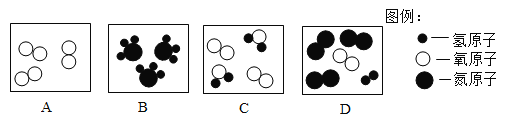
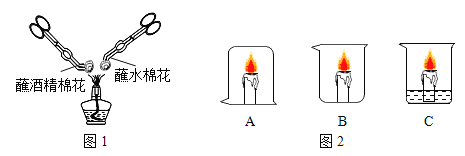
�ٴ��۽Ƕȿ���Bͼ��ʾ��__���ѧ���ţ��ڴӺ�۽Ƕȿ���ͼ�б�ʾ��������__������ĸ���ţ�
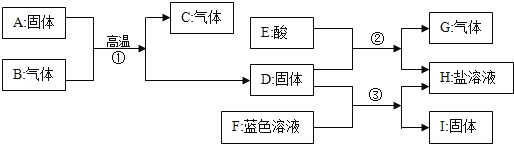
��2����һ�������£�A��B�ܷ�����ѧ��Ӧ����E��F������ʾ��ͼ������ʾ��
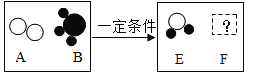
����FΪ��������������������壬��÷�Ӧ�����ɵ�E��F��������__��
����FΪ�������A��B�ķ��Ӹ�����Ϊ5��4����÷�Ӧ�Ļ�ѧ����ʽΪ__��
���𰸡�NH3 CD 27��14 5O2+4NH3 6H2O+4NO
6H2O+4NO
��������
��1������B���ӵĹ��ɿ�֪��Bͼ��ʾ��������ѧʽ�ǣ�NH3��
����A��B��C��D��ʾ�������ʵ���ʾ��ͼ��֪����C��D�ж����ɲ�ͬ�ַ��ӹ��ɵ����ʣ��Ӻ�������ɲ�ͬ��������ɵģ����ڻ���
��2�����������֪����һ�������£�A��B�ܷ�����ѧ��Ӧ����E��F����FΪ��������������������壬ӦΪ�������ɷ�Ӧ����ʾ��ͼ��֪���÷�Ӧ�ǰ�����������һ�������·�Ӧ����ˮ�͵���������ʽΪ��4NH3+3O2 6H2O+2N2���ɷ���ʽ��֪��E��F��������Ϊ����6����1��2+16��������2��14��2��=27��14��
6H2O+2N2���ɷ���ʽ��֪��E��F��������Ϊ����6����1��2+16��������2��14��2��=27��14��
�����������غ㶨�ɿ�֪Fһ���ǵ����������AB�ķ��Ӹ�����5��4������жϳ�FΪһ������������д������ʽ�ǣ�5O2+4NH3 6H2O+4NO��
6H2O+4NO��

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�