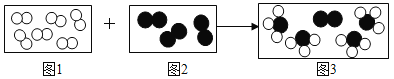
��Ŀ����
����Ŀ��ľ̿��ԭ����ͭʵ���Ļ�Ϸ�ĩ�к���ͭ������ͭ��ľ̿�ۣ�ij��ѧʵ��С����ƻ���ͭ�ķ�������:
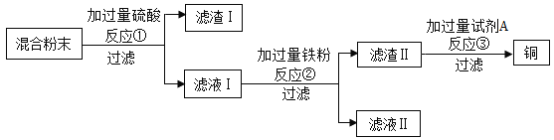
(1)���˲����б����õ��IJ����������ձ�����������___________________�����в�������������__��
(2)��Ӧ�ٵĻ�ѧ����ʽΪ__________________��
(3)��ҺII�е���������Ϊ___________________��
(4)�Լ�A���ѡ������_______________��Һ(�����)��
��H2SO4 ��CuSO4 ��MgSO4
(5)Ϊ������ʵ�鷽�����ɶ�����I�е�______________���л��ա�
���𰸡�©�� ���� CuO+H2SO4=CuSO4+H2O �������� A ͭ
��������
��1�����˲����б����õ��IJ����������ձ�����������©�������в�������������������
��2��ͭ��ľ̿������ܷ�Ӧ���������̿�֪����Ӧ��������ͭ�����ᷴӦ��������ͭ��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CuO+H2SO4=CuSO4+H2O ��
��3����Һ���м���������ۣ�����ͭ�����۷�Ӧ��������������ͭ����������������۷�Ӧ�������������������������Һ���к��е�����������������
��4��A���������к���ʣ�������ͭ���������ᷴӦ��������������������ͭ�������ᷴӦ����������ɳ�ȥ������������ʱ�������������˵������ȫ��ȥ������õķ������������⣻
B������ͭ�������뷴Ӧ���ɳ�ȥ������������ȷ�����Ƿ���ȫ��ȥ����������õķ��������������⣻
C������þ����������Ӧ�����ܳ�ȥ�������������⡣��ѡA��
��5����ʼ��Ϸ�ĩ�е�ͭû�к����ᷴӦ�������������У�Ϊ�˻��ո����ͭ���ɽ�����1�е�ͭҲ���գ�Ϊ������ʵ�鷽�����ɶ�����I�е�ͭ���л��ա�

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�����Ŀ������ʵ�������ͼ��ʾ�仯���Ƶ��ǣ�������
��� | ʵ�� | ������ | ������ |
A | ��һ��������������Һ�еμ�ϡ���� | ϡ��������� | ˮ������ |
B | ����һ����������ع��� | ʱ�� | ʣ���������Ԫ�ص����� |
C | ��һ���������������Һ�м�ˮ | ��ˮ������ | ���ʵ��������� |
D | ��һ����þ���м���ϡ���� | ϡ��������� | þ�۵����� |

A.AB.BC.CD.D