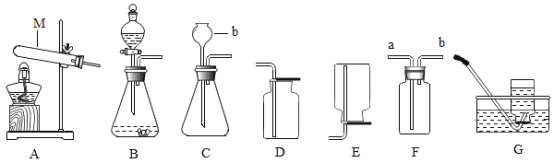
��Ŀ����
����Ŀ��ʵ���ҳ��õĸ��������ʯ������CaO����NaOH�Ļ����,�����������ˮ������CO2��Ӧ������ijͬѧ��һƿ���õ�����ʯ������������̽��������֪��Ca(OH)2+Na2CO3=CaCO3��+2NaOH��
��1�����룺
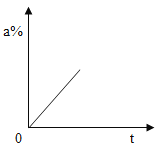
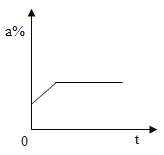
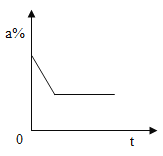
��������û�б���,����ʯ����ֻ����CaO������NaOH��
������������ȫ����,����ʯ����ȫ�������CaCO3��Na2CO3��
��CaO��ˮ��Ӧ�ķ�Ӧ����ʽΪ_________��
��2��ʵ�飺��ͼ��ʾ��
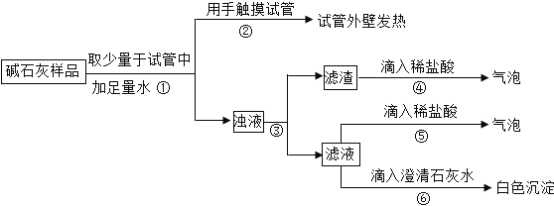
��3���жϣ�
a��Ca(OH)��CaCO3��Na2CO3Ͷ�뵽ˮ�в������,���ɲ����������жϣ�������______�����������������
b NaOH��¶�ڿ����б��ʵĻ�ѧ����ʽΪ______���ɲ����ݢ������жϣ���Һ�к���______��д��ѧʽ�����ɴ��жϲ�����______�������������������
c�ۺ�a��b�Ľ���,�жϸ���Ʒ�������Ϊ______��
��4����չ��������ʵ��˵��,ʵ����������ʯ����Ӧ______���棻�������в�������������______
���𰸡�CaO+H2O=Ca(OH)2 ������ 2NaOH+CO2=Na2CO3+H2O Na2CO3 ������ ���ֱ��� �ܷ� ����
��������
��1�����룺CaO��ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca(OH)2�����CaO+H2O=Ca(OH)2��
��3���жϣ�a���ڲ����ڵ������Ƿ��ȣ���ô�����������ƺ�ˮ��Ӧ���ȣ���NaOH����ˮ���ȵ��µģ�˵����Ʒ�к��������ƣ����������ƣ��������ƺ��������ƣ�˵����Ʒû����ȫ���ʣ��������������������������
b NaOH��¶�ڿ�����������еĶ�����̼��Ӧ������̼���ƺ�ˮ����˱��ʵĻ�ѧ����ʽΪ2NaOH+CO2=Na2CO3+H2O���ɲ����ݣ�����ϡ���������ݲ�����˵�����溬�п����Ե�̼���Σ��������֪��Һ�к���̼���ƣ�����Һ�к��е�̼���ƺ�ʯ��ˮ��Ӧ���ɲ�����ˮ��̼��ƺ�ˮ������֤������Һ���溬��̼������Һ��֤���˲����������������2NaOH+CO2=Na2CO3+H2O��Na2CO3����������
c��a�ó��Ľ��ۣ�ȫ�������Ǵ���ģ���b�ó��Ľ����ǣ�û�б����Ǵ���ģ��ۺ�a��b�Ľ��ۣ��жϸ���Ʒ�������Ϊ���ֱ��ʣ����ʵ�ԭ�����������ƺͿ����еĶ�����̼��Ӧ����̼���ƺ�ˮ��������ֱ��ʡ�
��4����ʯ���ڿ����м��ױ��ʣ�������Ҫ�ܷⱣ�棬����ʱ��Һ������⽦����Ҫ������������������ܷ⣻������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��Ϊ��̽���������������طֽ��ٶȵ�Ӱ��,ij��ȤС������ͬ�ļ��������������ɵ����������Ϊ��,�������������ʵ�飺
������ | ���������/g | ���� | ��������/g | �ռ�50mL��������ʱ��/s |
1 | 5 | _____ | _____ | 171 |
2 | X | �������� | 0.5 | 49 |
3 | 5 | ������ | 0.5 | 58 |
4 | 5 | �Ȼ��� | 0.5 | 154 |
��1��д��������ڶ������̵Ĵ������·�����Ӧ�����ֱ���ʽ_____��
��2��ʵ�����,X��ֵΪ_____�Ա�ʵ��ں�ʵ���,���Եó��Ľ�����:_____�Ĵ�Ч�����á�
��3���Ա�ʵ��ٺ�ʵ��ܿ�֪�Ȼ���_____����С����ޡ��������á�ά�ּ�����������,��ʵ����ټ����ռ�50mL����,����ʱ������С��171s,ԭ����_____��
��4��Ҫ�Ƚϴ������������طֽ��ٶȵ�Ӱ��,���˲����ռ�50mL���������ʱ�䣬�����Բ���_____��
����Ŀ����1�����Ϲ��� 2019 ����Ϊ�����ʻ�ѧԪ�����ڱ��ꡱ���ȡ�þԪ����Ԫ�����ڱ��еIJ�����Ϣ��ԭ�ӽṹʾ��ͼ���£���ش��������⡣
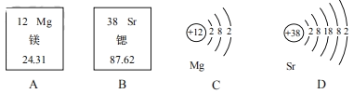
����ԭ���ڻ�ѧ��Ӧ������_____��ѡ��á���ʧ�������ӣ��ȵ�������Ļ�ѧʽΪ_____��
��Ԫ�ص���������ԭ�ӽṹ�����й�ϵ��ԭ�ӵ�������������ͬʱ�����Ӳ���Խ�࣬�����������ԽԶ��ԭ�Ӻ˶��������ӵ�������ԽС���ɴ��Ʋ⣬��ԭ�ӵ�ʧ���ӵ�������þ��_____��ѡ�ǿ������������
��2���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ��ѧϰ���ص㡣�ס��ҡ���������ʾ�������ʣ����ǵ���ʾ��ͼ�����ʾ
���� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
|
��һ�������ӹ���_____��ԭ�ӡ�
�ڼס�������������һ�������·�Ӧ���ɱ��Ͷ�����Ӧ�Ļ�ѧ����ʽΪ_____���μӷ�Ӧ�ļ��ҵķ��Ӹ�����Ϊ_____��