��Ŀ����
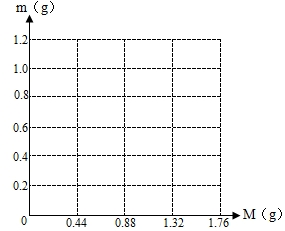
С���ڻ�ѧʵ���ҷ��֣�ʢ���ռ������Լ�ƿƿ��δ��¶���ڿ����У�С�ս���С����С�죬��ͬ̽�������ռ���Ƿ���ʣ�Ϊ�ⶨ��ɷ֣�С����ȡ���ռ���Ʒ3g������ܽ���ˮ�У��ٵμ�����������Һ�������������������������������Һ��������ϵ����ͼ��ʾ����
��1�������ij�����______���ѧʽ����
��2�����ռ���Ʒ���������Ƶ�������������С������λ��
��3������������������Һ����������������

��1�������ij�����______���ѧʽ����
��2�����ռ���Ʒ���������Ƶ�������������С������λ��
��3������������������Һ����������������

��1���������ƺ�̼��������̼��ƺ��������ƣ�̼����dz������ʴ�Ϊ��CaCO3��
��2����ͼ����Կ�����������������Һ�������ﵽ200�ˣ���������Ҳ�ﵽ���ֵ��0.3�ˣ�������Ʒ��̼���Ƶ�������X���μӷ�Ӧ���������Ƶ�������Y��
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
74 106 100
Y X 0.3��
�T
�ã�Y=0.22��
�T
�ã�X=0.32��
���ռ���Ʒ���������Ƶ�����=3��-0.32��=2.68�ˣ�
��3���ɣ�2��������������Ƶ�������0.22�ˣ���ͼ����Կ���������������Һ��������200�ˣ���������������Һ�����ʵ���������=
��100%�T0.11%��
�𣺸��ռ���Ʒ���������Ƶ�����Ϊ2.68�ˣ�����������������Һ��������������Ϊ0.11%��
��2����ͼ����Կ�����������������Һ�������ﵽ200�ˣ���������Ҳ�ﵽ���ֵ��0.3�ˣ�������Ʒ��̼���Ƶ�������X���μӷ�Ӧ���������Ƶ�������Y��
Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
74 106 100
Y X 0.3��
| 74 |
| 100 |
| Y |
| 0.3�� |
�ã�Y=0.22��
| 106 |
| 100 |
| X |
| 0.3�� |
�ã�X=0.32��
���ռ���Ʒ���������Ƶ�����=3��-0.32��=2.68�ˣ�
��3���ɣ�2��������������Ƶ�������0.22�ˣ���ͼ����Կ���������������Һ��������200�ˣ���������������Һ�����ʵ���������=
| 0.22�� |
| 200�� |
�𣺸��ռ���Ʒ���������Ƶ�����Ϊ2.68�ˣ�����������������Һ��������������Ϊ0.11%��

��ϰ��ϵ�д�
�����Ŀ