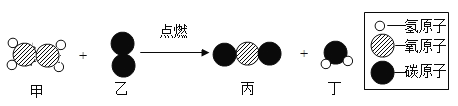
��Ŀ����
����Ŀ����������ijһ��Ԫ�ص�ԭ�Ӷ�ȱ���γɷ����Ȼ������������и���ԭ�ӵ������Ŀ�����ü������ȱ�ʾ����Fe0.9O��PbO1.9�ȡ������Ȼ����������͵Ĺ��ܲ��ϣ����кܴ�ĿƼ���ֵ��
��1��PbO1.9��ǦԪ�ؾ���+2��+4 ���ּ�̬������+4 ��ǦռǦ������_______��
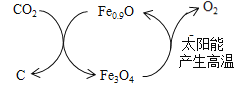
��2����ͼ��ʾ��Fe0.9O������CO2���ۺ����á�
����д����ת���������ŵ㣺___________��__________��
������1mol Fe0.9O��������CO2��ȫ��Ӧ������_g C��̼����
���𰸡�90% ������̫���ܽ�CO2ת��ΪC��O2 Fe0.9O��ѭ��ʹ�� 1.2
��������
��1��PbO1.9��ǦԪ�ؾ���+2��+4 ���ּ�̬����+4 ��ǦռǦ������x�����ݻ������Ԫ�ػ��ϼ۴�����Ϊ�㣬��+4x+2����1-x��-2��1.9=0��x=0.9����+4 ��ǦռǦ������90%��
��2���ٸ���ͼʾ��֪��Fe0.9O���Խ�CO2ת��ΪFe3O4��C����Fe3O4����̫���ܸ��������·ֽ�ΪFe0.9O���������ڴ�ѭ�������У�������̫���ܣ�Fe0.9O��ѭ��ʹ�ã����ܽ�CO2ת��ΪC��O2�������˿�����Ⱦ����ת���������ŵ㣺������̫���ܽ�CO2ת��ΪC��O2��Fe0.9O��ѭ��ʹ�á�
����![]() ��֪��1mol Fe0.9O��������CO2��ȫ��Ӧ������0.1mol̼������Ϊ
��֪��1mol Fe0.9O��������CO2��ȫ��Ӧ������0.1mol̼������Ϊ![]() ��
��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ��Ϊ�ⶨ�����ڿ����е�����������Ʒ�ı��ʳ̶ȣ�ij��ѧ�С���ȡ�ù�����Ʒ6.5g������ƿ�У���ˮ�ܽ⣬���50g��Һ��������ƿ�еμ�ϡ���ᣬ��Ӧ�����в������������IJ���ʵ�����ݺ���ƿ����Һ�����仯��ͼ�������ʾ��
����ϡ���������/g | 40 | 65 | 75 |
�������������/g | 1.1 | 2.2 | 2.2 |
(1)6.5g��Ʒ��ϡ������ȫ��Ӧ���������������________g��
(2)6.5g��Ʒ��̼���Ƶ�����������________��(�����ȷ��0.1%)
(3)�������ε�ʵ�������У�ֻ��һ�μ����ϡ��������ƿ����Һ������ǡ����ȫ��Ӧ��
����ͼͼ����a����ֵ��________��
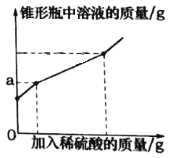
�����ʵ��������ϡ�����������������____��(д���������)
����Ŀ����ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2 ��H2O��g������ˮú���������в���ת��Ϊ�ϳɰ���ԭ��N2��H2��
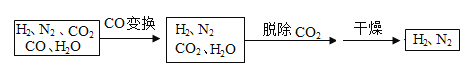
��1����ʹ��ͭ�����ͼ��������£���ˮú����Ҫ�ɷּ䷢����Ӧʵ���� CO �任���仯ѧ����ʽΪ��________��
��2�����շ����ѳ�CO2�ķ���֮һ����֪��
���� | Na2CO3 | K2CO3 |
20��1L������Һ�����ʵ����ʵ���mol | 2.0 | 8.0 |
���ʼ۸�Ԫ/kg�� | 1.25 | 9.80 |
��ѡ��Na2CO3��Һ������Һ�����ŵ���_��ȱ����_�����ѡ��K2CO3��Һ������Һ����ij�ַ������Խ��ͳɱ���д�����ַ����漰�Ļ�ѧ����ʽ��_��
��3����һ�������ˮú������ͨ��װ�â�~�������ͨ�뵪��ȷ����Ӧ��������ȫ�������� �ⶨ����H2�Լ� CO �����ʵ�����
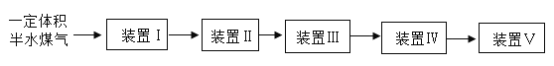
�ɹ�ѡ�õ�װ��������ʾ��ʡ�Լг���������
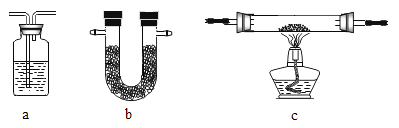
Ϊװ�â�ѡ����ʵ�װ�����Լ���
װ�� | �� | �� | �� | �� | �� |
a | a | _ | _ | b | |
�Լ� | _ | Ũ���� | CuO | _ | _ |
װ�â��������____________��Ҫȷ����ˮú����H2���ʵ�����Ӧ������������______��
����Ŀ����ͭ��ͭ��п�ĺϽ������ƽ�ʮ�����ƣ�����ͭ�ڳ�ʪ�Ŀ����г�ʱ����û����ͭ�̣����ƽ�Ͳ��ᡣij��ѧ��ȤС���ͬѧ����һ��������ͭ�̵���Ʒչ����̽�����
[ʵ��̽��]����С������ͭ�̣�����������������ڼ��ȣ������˺�ɫ��������ͭ��ˮ��ʹ����ʯ��ˮ����ǵ�����ζ���塣
��ȡһС�������ͭ�̵Ļ�ͭƬ����ϡ�����У�����ͼA�����۲쵽�����ݲ�������Һ������ͨ��ʵ��̽���ٿ�֪ͭ�̵ijɷ�����_____��Ԫ����ɡ�
[�������1 ]ʵ��̽���������ɵ�������ʲô?
[���������]
����һ:���ɵ�������CO2
�����:���ɵ�������H2
������:���ɵ�������_______
[ʵ����֤]
ͬѧ��������������ͼ��ʾ��װ�ý�����֤:
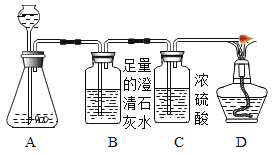
A�з�Ӧ�ϳ�ʱ����ٵ�ȼD���ľƾ��ơ��۲쵽________������֤������������������A����Ʒ�м���ϡ�����������ȼD���ľƾ��ƣ�������ɵĽ����________��
[�������2] A�з�Ӧ����Һ����ɫ��������������Щ?
ijͬѧ��Ϊ��ɫ��Һ�п��ܺ���CuSO4��H2SO4��
[ʵ����֤]Ϊ֤����ɫ��Һ�п��ܴ���CuSO4��H2SO4��ͬѧ�ǽ���������ʵ�顣
ʵ����� | ʵ������ | ʵ����� |
ȡ������ɫ��Һ���Թ��У����������ĥ����пƬ | ____ | ������һ������H2SO4 |
_____ | ������һ������CuSO4 |
����Ϊ������Ӧ����Һ�л����ڵĽ���������_______��д���ӷ��ţ���
[��չ����]
�в��������û�ͭð��ƽ���ƭ������������������ַ����������ٻƽ�
��� | ���� | ���� | ���� |
1 | _____ | _____ | Ϊ�ٻƽ� |
2 | _____ | _____ |