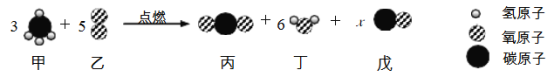��Ŀ����
����Ŀ��д�����з�Ӧ�Ļ�ѧ����ʽ��
��1������ƽ�����ʢ��һ����ɫ��״Һ��������N2H4����˫��ˮ�������ǻ��ʱ����������ˮ������һ �ֵ��ʣ����ų��������ȡ��䷴Ӧ�Ļ�ѧ����ʽΪ��___��
��2���״���CH3OH����һ�־綾�ġ����оƾ���ζ��Һ�塣��һ�����ļ״��� 4.4g ��������һ���ܱ��� ���ڣ���ȼ����ַ�Ӧ������Ӧ����ȫת��Ϊ�����������Ϊһ����̼��������̼��ˮ������ȴ���� �£��������������ˮ������Ϊ 3.6g���䷴Ӧ�Ļ�ѧ����ʽΪ��______ ��
���𰸡�N2H4 2H2O2 4H2O N2 8CH3OH 11O2 ![]() 2CO 6CO2 16H2O
2CO 6CO2 16H2O
��������
��1��������N2H4����˫��ˮ��H2O2�������ʱ����ˮ������һ�ֵ��ʣ����������غ㶨�ɣ����ɵĵ����ǵ�������Ӧ�Ļ�ѧ����ʽΪ��N2H4 2H2O2 4H2O N2 ��
��2��3.6gˮ����Ԫ�ص�����=![]() ����Ԫ�ص�����=3.6g-0.4g=3.2g�����������غ㶨�ɿ�֪��ˮ�е���Ԫ��ȫ�����Լ״�����״�������=
����Ԫ�ص�����=3.6g-0.4g=3.2g�����������غ㶨�ɿ�֪��ˮ�е���Ԫ��ȫ�����Լ״�����״�������=![]() ���״���̼Ԫ�ص�����=
���״���̼Ԫ�ص�����=![]() ����״�����Ԫ�ص�����=3.2g-0.4g-1.2g=1.6g�����������غ㶨�ɿ�֪�� һ����̼�Ͷ�����̼��̼Ԫ�ص�����=1.2g����Ԫ�ص�����=1.6g+4.4g-3.2g=2.8g���������̼������Ϊm��һ����̼����Ϊn�����У�
����״�����Ԫ�ص�����=3.2g-0.4g-1.2g=1.6g�����������غ㶨�ɿ�֪�� һ����̼�Ͷ�����̼��̼Ԫ�ص�����=1.2g����Ԫ�ص�����=1.6g+4.4g-3.2g=2.8g���������̼������Ϊm��һ����̼����Ϊn�����У�
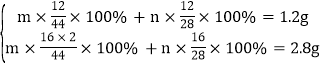
��֮�ã�m=3.3g ��n=0.7g�����������֪���״���������Ӧ����һ����̼��������̼��ˮ���裺�÷�Ӧ�Ļ�ѧ����ʽΪ��xCH3OH+yO2![]() mCO2+nCO+zH2O������
mCO2+nCO+zH2O������![]() ��
��![]() ��
��![]() ��
��![]() ������������x��y��m��n��z=8��11��6��2��16�����Ը÷�Ӧ�Ļ�ѧ����ʽΪ��8CH3OH 11O2
������������x��y��m��n��z=8��11��6��2��16�����Ը÷�Ӧ�Ļ�ѧ����ʽΪ��8CH3OH 11O2 ![]() 2CO 6CO2 16H2O��
2CO 6CO2 16H2O��