��Ŀ����
����Ŀ��ij��ѧ��ȤС����в�����������(FeC2O42H2O)�ֽ��ʵ��̽����
���� �롿������������ֽ�����CO��CO2��H2O�������塣
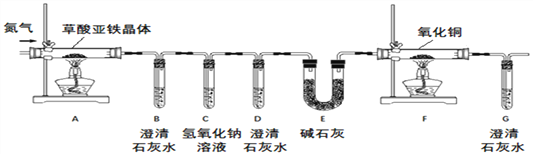
��ʵ�鷽����������ͼװ�ý���ʵ��(�г�װ��δ����)��
���������ۡ�(1)ʵ�鿪ʼǰ��Ҫ�ȹ���һ��ʱ���N2���ò�����Ŀ��Ϊ_________��
(2)C������������Һ��������_______��
(3)E�м�ʯ�ҵ�������___________��
�����������(4)���Է�����
���Թ�D�г��ֻ��ǣ�֤����������_____���ڣ�֤���ֽ�����д���CO��������_____��
��С����ΪӦ����Hװ�ã���Hװ��Ӧ����____����װ��֮�䣬���۲쵽____����֤����ˮ���ɣ�
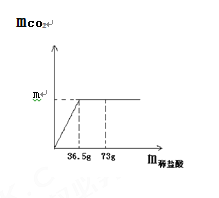
(5)��������(�ٶ�ÿһ����Ӧ������ȫ)��ȡ3.6g��Ʒ��������ʵ�飬���װ��AӲ�ʲ������в���1.44g��ɫ����FeO��װ��F��Ӳ�ʲ����ܹ�����������0.32g���������������(FeC2O42H2O)�ֽ�õ���CO2������Ϊ______��
����˼���ۡ�(6)�ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����______��
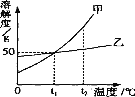
(7)������ʾ��FeC2O42H2O���ȷֽ�ʱ��������������¶ȱ仯����������ͼ��ʾ��д�����ȵ�400oCʱ��FeC2O42H2O���ȷֽ�Ļ�ѧ����ʽ_______��
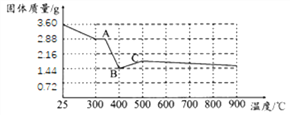
����ͼ������3.6gFeC2O42H2O�ڳ��ڻ����г�ּ��ȣ����յõ�����ɫ����1.60g��������ʵĻ�ѧʽΪ_______���ɴˣ�����Ϊ���и�ʵ����Ҫע���������________________��
���𰸡� �ž�װ���ڵĿ�������ֹ�����е�CO2�ȶ�ʵ����ɸ��� ����CO2����ֹ��CO�ļ����γɸ��� ����ˮ����(�����CO����) CO2 D��ʯ��ˮ������ǣ�G��ʯ��ˮ�����(���ɫ������) AB ��ˮ����ͭ���� 0.88g û�д���β�� FeC2O42H2O![]() FeO+CO��+CO2��+2H2O�� Fe2O3 FeC2O42H2O�ֽ�ʵ��Ӧ���ܱջ����н��У���ΪFeO�ױ�������
FeO+CO��+CO2��+2H2O�� Fe2O3 FeC2O42H2O�ֽ�ʵ��Ӧ���ܱջ����н��У���ΪFeO�ױ�������
�������������ڲ�����������(FeC2O42H2O)�ֽ��ʵ��̽�����龳�¿�����CO2��CO��H2O�ļ��飬���ݻ�ѧ����ʽ���ۺϼ��㣬�ۺϽ�ǿ��Ū��ÿ��װ�õ����úͷ�Ӧ���ߵĺ����ǽ���Ĺؼ�����ѧ����������Ԫ�ص��غ�Ĺ��
(1) �����е�CO2�ȶ�ʵ���и��š�ʵ�鿪ʼǰ��Ҫ�ȹ���һ��ʱ���N2���ò�����Ŀ��Ϊ�ž�װ���ڵĿ�������ֹ�����е�CO2�ȶ�ʵ����ɸ��ţ�
(2)CO��ͨ������ͭ�ı�ɫ������ʯ��ˮ�����������ġ�C������������Һ������������CO2����ֹ��CO�ļ����γɸ��ţ�
(3)E�м�ʯ�ҵ�����������ˮ����(�����CO����)��
(4)��CO2��ʹ�����ʯ��ˮ����ǡ��Թ�D�г��ֻ��ǣ�֤����������CO2���ڣ�����ͭ��һ����̼��Ӧ����ͭ�Ͷ�����̼��֤���ֽ�����д���CO��������D��ʯ��ˮ������ǣ�G��ʯ��ˮ�����(���ɫ������)��
��С����ΪӦ����Hװ�ã���Hװ��Ӧ����AB����װ��֮�䣬���۲쵽��ˮ����ͭ��������֤����ˮ���ɣ�
(5)���ݻ�ѧ����ʽCuO +CO �� Cu + CO2��֪װ��F��Ӳ�ʲ����ܹ������������0.32g�Dzμӷ�Ӧ������ͭ����Ԫ�ص�������
�裺�μӷ�Ӧ��CO������Ϊx
CuO +CO �� Cu + CO2 ��m
28 16
X 0.32g
![]() x=0.56g
x=0.56g
3.6g�IJ�����������(FeC2O42H2O)��FeC2O4������=3.6g��![]() ��100%=2.88g��FeC2O4�ֽ������FeO��CO��CO2�����Բ�����������(FeC2O42H2O)�ֽ�õ���CO2������=2.88g-0.56g-1.44g=0.88g��
��100%=2.88g��FeC2O4�ֽ������FeO��CO��CO2�����Բ�����������(FeC2O42H2O)�ֽ�õ���CO2������=2.88g-0.56g-1.44g=0.88g��
(6)һ����̼�ж���ֱ���ŷŵ������л���Ⱦ�������ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����û�д���β����
(7) ���ȵ�400oCʱ��FeC2O42H2O��ȫ�ֽ⣬��ѧ����ʽ��FeC2O42H2O![]() FeO+CO��+CO2��+2H2O����
FeO+CO��+CO2��+2H2O����
����1.44g��FeO����Ԫ�ص�����=1.44g��![]() ��100%=1.12g�� ���������غ㶨���ڻ�ѧ�仯����Ԫ�ص��������䣬����ɫ��������Ԫ�ص�����=1.60g-1.12g=0.48g���裺��ɫ����Ļ�ѧʽΪFeaOb������56a��16b=1.12g��0.48g=2��3�����Ժ�ɫ����Ļ�ѧʽΪFe2O3�����������ڿ����м�������������������ʵ����Ҫע���������FeC2O42H2O�ֽ�ʵ��Ӧ���ܱջ����н��У���ΪFeO�ױ������ȡ�
��100%=1.12g�� ���������غ㶨���ڻ�ѧ�仯����Ԫ�ص��������䣬����ɫ��������Ԫ�ص�����=1.60g-1.12g=0.48g���裺��ɫ����Ļ�ѧʽΪFeaOb������56a��16b=1.12g��0.48g=2��3�����Ժ�ɫ����Ļ�ѧʽΪFe2O3�����������ڿ����м�������������������ʵ����Ҫע���������FeC2O42H2O�ֽ�ʵ��Ӧ���ܱջ����н��У���ΪFeO�ױ������ȡ�

 ��У����ϵ�д�
��У����ϵ�д�