��Ŀ����
С���ҹ�����һƿ�״ף���ǩ��ע�����������������5%����ƿ�״��д���ĺ����Ƿ����ǩ�ı�ע�����������С��һ�����й�����֪ʶ�������ⶨ�״��д���ĺ�����
��ʵ��ԭ����
��1������֪Ũ�ȵ�����������Һ����Һ���ʹ��ᣨ����Һ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+NaOH=CH3COONa+H2O
��2���ڻ����Һ�У������������������ȫ�к�ʱ��������1������������Һ����Һ�ͳʼ��ԣ���1������������Һ���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
��ʵ�鲽�衿��ȡ12.0mL�״ף��ܶȽ���Ϊ1.0g/mL���������ձ��У��ټ���20mL����ˮϡ�ͣ�
����ȡ45.0mL������������Ϊ1.0%������������Һ���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι�ȡ������������Һ����εصμӵ�ϡ�ͺ�İ״��У�ͬʱ���ϵؽ����ձ��е���Һ������ǡ����ȫ��Ӧ��ʣ������������Һ5.0ml��
��������˼��
��1����ʵ��������¶���α仯 ��
��2����ʵ�鲽������ڽ���������������������NaOH��Һ��ǡ����ȫ��Ӧ��ʵ������ ������С�����䡱��
��3����ʵ�鲽����У�С�����ȷ�������������������ȫ�кͣ� ��
��4��������ʵ�鲽����з���������ð�������������ԭ�� ��
�����ݴ���������ʵ�����ݣ�ͨ�������жϰ״��д���ĺ������ǩ�ı�ע�Ƿ������
��ʵ��ԭ����
��1������֪Ũ�ȵ�����������Һ����Һ���ʹ��ᣨ����Һ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+NaOH=CH3COONa+H2O
��2���ڻ����Һ�У������������������ȫ�к�ʱ��������1������������Һ����Һ�ͳʼ��ԣ���1������������Һ���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
��ʵ�鲽�衿��ȡ12.0mL�״ף��ܶȽ���Ϊ1.0g/mL���������ձ��У��ټ���20mL����ˮϡ�ͣ�
����ȡ45.0mL������������Ϊ1.0%������������Һ���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι�ȡ������������Һ����εصμӵ�ϡ�ͺ�İ״��У�ͬʱ���ϵؽ����ձ��е���Һ������ǡ����ȫ��Ӧ��ʣ������������Һ5.0ml��
��������˼��
��1����ʵ��������¶���α仯
��2����ʵ�鲽������ڽ���������������������NaOH��Һ��ǡ����ȫ��Ӧ��ʵ������
��3����ʵ�鲽����У�С�����ȷ�������������������ȫ�кͣ�
��4��������ʵ�鲽����з���������ð�������������ԭ��
�����ݴ���������ʵ�����ݣ�ͨ�������жϰ״��д���ĺ������ǩ�ı�ע�Ƿ������
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,�й��������������ļ���,�кͷ�Ӧ����Ӧ��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
�����������漰����������кͷ�Ӧ�ļ������⣬����кͷ�Ӧ�ų�����������кͷ�Ӧ�ζ��յ���жϷ����Ǹ��ݵζ�������ָʾ����ɫ�ı仯���жϵģ������ǵ������һ������������Һʱ���ձ���Һ������ɫ���ɫ��
����⣺��1������кͷ�Ӧ�ų��������ʴ�Ϊ���¶����ߣ�
��2����������кͷ�Ӧ��ʵ�ʣ�����֪������Һ����������ʹ���ʴ�����������٣�ʹ���к������������Ƶ������٣����������Ƽ�����Ĵ��������֮���٣��ʴ�Ϊ����С��
��3�������Dzⶨ�״��д���ĺ���������Ҫ���뼸�η�̪��Һ��ָʾ������̪��Һ�����ɫ��������ɫ������ָʾ����ɫ�ı仯���жϷ�Ӧ�Ľ���������ʴ�Ϊ���������һ������������Һʱ���ձ���Һ������ɫ���ɫ��
��4������������������еĶ�����̼���巴Ӧ����̼���ƣ�̼�������ᷴӦ��ų�������̼���壻
�����ݴ������������йػ�ѧ����ʽ�ļ��㲽�裺һ�衢��д�����ꡢ���С�������ǿ�����ɴ��⣬���ڼ�����x=5����ǩ��ע�����������������5%���Ӷ��жϣ���ƿ�״��д���ĺ������ǩ�ı�ע������ʴ�Ϊ��
�⣺��״��д������������Ϊx%
CH3COOH+NaOH�TH3COONa+H2O
60 40
12mL��1.0g/mL��x% ��45-5��mL��1.0g/mL��1.0%
60����12mL��1.0g/mL��x%��=40��[��45-5��mL��1.0g/mL��1.0%]
��ã�x=5
�𣺰״��д���������������ǩ�ı�ע�����
�ʴ�Ϊ����1���¶�����
��2����С
��3�����ڴ����еμӷ�̪���������������Һ����ɫ�պñ�ɺ�ɫʱ��ʾ��Ӧ��ȫ��
��4���������Ʊ��ʡ���
[���ݴ���]�⣺��״��д������������Ϊx%
CH3COOH+NaOH�TH3COONa+H2O
60 40
12mL��1.0g/mL��x% ��45-5��mL��1.0g/mL��1.0%
60����12mL��1.0g/mL��x%��=40��[��45-5��mL��1.0g/mL��1.0%]
��ã�x=5
�𣺰״��д���������������ǩ�ı�ע�����
��2����������кͷ�Ӧ��ʵ�ʣ�����֪������Һ����������ʹ���ʴ�����������٣�ʹ���к������������Ƶ������٣����������Ƽ�����Ĵ��������֮���٣��ʴ�Ϊ����С��
��3�������Dzⶨ�״��д���ĺ���������Ҫ���뼸�η�̪��Һ��ָʾ������̪��Һ�����ɫ��������ɫ������ָʾ����ɫ�ı仯���жϷ�Ӧ�Ľ���������ʴ�Ϊ���������һ������������Һʱ���ձ���Һ������ɫ���ɫ��
��4������������������еĶ�����̼���巴Ӧ����̼���ƣ�̼�������ᷴӦ��ų�������̼���壻
�����ݴ������������йػ�ѧ����ʽ�ļ��㲽�裺һ�衢��д�����ꡢ���С�������ǿ�����ɴ��⣬���ڼ�����x=5����ǩ��ע�����������������5%���Ӷ��жϣ���ƿ�״��д���ĺ������ǩ�ı�ע������ʴ�Ϊ��
�⣺��״��д������������Ϊx%
CH3COOH+NaOH�TH3COONa+H2O
60 40
12mL��1.0g/mL��x% ��45-5��mL��1.0g/mL��1.0%
60����12mL��1.0g/mL��x%��=40��[��45-5��mL��1.0g/mL��1.0%]
��ã�x=5
�𣺰״��д���������������ǩ�ı�ע�����
�ʴ�Ϊ����1���¶�����
��2����С
��3�����ڴ����еμӷ�̪���������������Һ����ɫ�պñ�ɺ�ɫʱ��ʾ��Ӧ��ȫ��
��4���������Ʊ��ʡ���
[���ݴ���]�⣺��״��д������������Ϊx%
CH3COOH+NaOH�TH3COONa+H2O
60 40
12mL��1.0g/mL��x% ��45-5��mL��1.0g/mL��1.0%
60����12mL��1.0g/mL��x%��=40��[��45-5��mL��1.0g/mL��1.0%]
��ã�x=5
�𣺰״��д���������������ǩ�ı�ע�����
�������������������ܹ�ץס�̲ĵ��ص㣬�Ӷ���Ƕȿ���ѧ���ķ����ͽ���������ӭ�����п��Ŀ��Է������п��Ŀ���֮һ��

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��������̼����̼��������ɵ� |
| B����������ɶ���Ԫ����ɵ� |
| C��ˮ����������ԭ�Ӻ�һ����ԭ�ӹ��ɵ� |
| D���������������ڵ��ʣ�������NH3���ڻ����� |
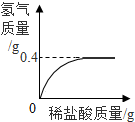 ����������̼�ĺϽ�Ϊ�ⶨij����ʯ������������Ʒ������������������ѧ��ȤС���ͬѧ�Ƶø�������Ʒ12.0g�������ձ��У������м���146gϡ����ʱǡ����ȫ��Ӧ�����ɵ����������ϡ�����������ϵ��ͼ��ʾ���������ʲ����뷴Ӧ��������㣺
����������̼�ĺϽ�Ϊ�ⶨij����ʯ������������Ʒ������������������ѧ��ȤС���ͬѧ�Ƶø�������Ʒ12.0g�������ձ��У������м���146gϡ����ʱǡ����ȫ��Ӧ�����ɵ����������ϡ�����������ϵ��ͼ��ʾ���������ʲ����뷴Ӧ��������㣺
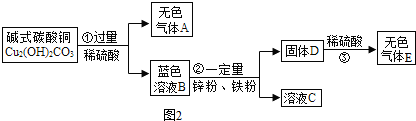

 X��Y���ֹ�����ܽ����ͼ��
X��Y���ֹ�����ܽ����ͼ��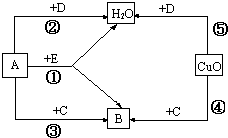 A��B��C��D��E�dz��л�ѧ�г�����5����ɫ���壬����2���ǵ��ʣ�3���ǻ��������EΪ��Ȼ������Ҫ�ɷ֣�����֮���ת����ϵ��ͼ��ʾ�����ƶϣ����ش��й����⣮
A��B��C��D��E�dz��л�ѧ�г�����5����ɫ���壬����2���ǵ��ʣ�3���ǻ��������EΪ��Ȼ������Ҫ�ɷ֣�����֮���ת����ϵ��ͼ��ʾ�����ƶϣ����ش��й����⣮