��Ŀ����
����Ŀ��ij�о���ѧϰС����������ȼ̼���ⶨ�����������������������ʵ��ʱ(��ͼ)�����ָ�����������������Һ�������ɵĶ�����̼��������ƿ��ˮ�����������ȻС��1/5��

��������⣩��ʲôԭ���²�����ȷ�أ�
����������裩���Ƿֱ��������²��룺
��ͬѧ��������ľ̿ȡ�������٣�
��ͬѧ��������ľ̿ȼ��û�������꼯��ƿ�е�������
��̽��һ��
С��ͬѧ����ʵ�������ȼ�ճ��л��в����ĺ�ɫ���壬����Ϊ_____ͬѧ�IJ��벻������
���������ϣ�ľ̿�������������ܱ�������ȼ��ֹͣ������������������ֱ�ߴ�14.0%��8.0%��16.0%
������ʵ�飩��ͬѧ����������װ�����������ʵ�飬������д�±���
ʵ�鲽�� | ʵ������ | ʵ������ |
�ٽ�����ľ̿��ȼ��Ѹ�ٲ��뼯��ƿ�� | _____ | ľ̿ȼ��û�������꼯��ƿ�е����� |
�ڴ�װ����ȴ��ȼ�ŵ�________(��������������)�����뼯��ƿ�� | _____ |
��ʵ�鷴˼��С����ľ̿ȼ�ղ���ĽǶȲ��뻹������������_____���²������С��1/5��������ע������ȡ����ƿ�ڵ���������ͼ2��ʾ��ʵ�飬���۲쵽����ʯ��ˮ����ǣ���֤��С���IJ����������������漰�Ļ�ѧ����ʽΪ_____����ʵ����û�й۲쵽����ͭ��ĩ����ԭ�������_____
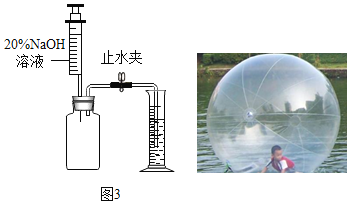
����չǨ�ƣ���Ҫ������ˮ�ϲ��������ڿ����ж�����̼���������������ͼ3װ�ã�������ƿ���ռ�����ˮ�ϲ��������ڿ�����Ʒ�����Ϊ500mL����������������������Һ�����ֹˮ�к���Ͳ�е�ˮ���뼯��ƿ��5mL�������ڿ�����CO2�����������_____
���𰸡���ľ̿ȼ��ʱ������⣬ȼ�IJ�������������ȼ��һ����̼CO2+2NaOH=Na2CO3+H2O CO+CuO![]() Cu+CO2CO2+Ca(OH)2=CaCO3��+H2Oʣ��ĺ�ɫ����ͭ�̫࣬ͭ��1%
Cu+CO2CO2+Ca(OH)2=CaCO3��+H2Oʣ��ĺ�ɫ����ͭ�̫࣬ͭ��1%
��������
(1)ľ̿����ɫΪ��ɫ����ȼ�ճ��л��в����ĺ�ɫ���壬˵��ľ̿��ʣ�࣬�ʼ�ͬѧ�������(2) ľ̿�ڿ�����ȼ�գ�������⣬ȼ�ղ�������(3)������Ŀ�в������Ͽ�֪��ľ̿�������������ܱ�������ȼ��ֹͣ������������������ֱ�ߴ�14.0%��8.0%��16.0%����ľ̿ȼ�����Ժ������������������Ϊ14.0%����ʹʣ�������������������Ҳ��ʹ����ȼ����Ӱ��ʵ����ۣ����ѡ������(4)���ݱ����е�ʵ����ۿ�֪��ֻ��������ȼ�գ����ܵó�ľ̿ȼ��û�������꼯��ƿ�е������Ľ��ۣ�����������ȼ�գ�(5)ľ̿��ȫȼ�����ɶ�����̼������ȫȼ��ʱ����һ����̼������һ����̼��(6) ʢ������������Һ���Թ��еĻ�ѧ��Ӧ����ʽΪCO2+2NaOH=Na2CO3+H2O��ʢ����������ͭ�IJ������л�ѧ��Ӧ����ʽΪ CO+CuO![]() Cu+CO2��ʢ�г���ʯ��ˮ�Թ��еĻ�ѧ��Ӧ����ʽΪCO2+Ca(OH)2=CaCO3��+H2O��(7)����ͭΪ��ɫ���壬ͭΪ��ɫ���壬��Ӧ��۲첻����ɫ������֣�ԭ�������������������������۲죬����ʣ��ĺ�ɫ����ͭ�࣬���ɵ�̫ͭ�٣�(8) ��ֹˮ�к���Ͳ�е�ˮ���뼯��ƿ��5mL��ˮ�������Ϊ������̼�����������ɵ����ڿ�����CO2�����������1%��
Cu+CO2��ʢ�г���ʯ��ˮ�Թ��еĻ�ѧ��Ӧ����ʽΪCO2+Ca(OH)2=CaCO3��+H2O��(7)����ͭΪ��ɫ���壬ͭΪ��ɫ���壬��Ӧ��۲첻����ɫ������֣�ԭ�������������������������۲죬����ʣ��ĺ�ɫ����ͭ�࣬���ɵ�̫ͭ�٣�(8) ��ֹˮ�к���Ͳ�е�ˮ���뼯��ƿ��5mL��ˮ�������Ϊ������̼�����������ɵ����ڿ�����CO2�����������1%��

 ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�