��Ŀ����
��ѧ������ʦ������һ��Сħ���������������С�����ͬ�����£���A��B���������ͬ�ļ���ƿ�зֱ������ɫ������(H2S)����Ͷ����������塣��ͼ��ʾ���鿪ë����Ƭ��ƿ�ڶԽ����ߵ����Σ�ʹ���������ֻ�ϡ�һ������۲쵽��ƿ�ڱڸ��ŵ���ɫ�����������ش��������⣺

(1)���á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��
����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��

�÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________________�����⡢������������Ԫ�صĻ��ϼ۷ֱ�Ϊ______________________��
(2)���������ƿ�����ʵؽ���������ƿ�����ǽ���۲쵽����ƿ����ˣ�ԭ����
______________________________________________________________________________��
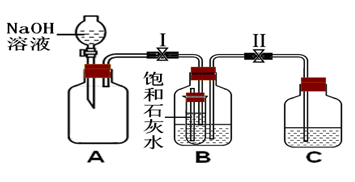
(3)��֪����ͬ�����£������������������ķ�����Ŀ��ȡ���Ӧ��ϣ�ƿ��ʣ�������Ϊ____________������������ķ�����_____________________________________��

(1)���á�
 ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��
����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��
�÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________________�����⡢������������Ԫ�صĻ��ϼ۷ֱ�Ϊ______________________��
(2)���������ƿ�����ʵؽ���������ƿ�����ǽ���۲쵽����ƿ����ˣ�ԭ����
______________________________________________________________________________��
(3)��֪����ͬ�����£������������������ķ�����Ŀ��ȡ���Ӧ��ϣ�ƿ��ʣ�������Ϊ____________������������ķ�����_____________________________________��
(1)2H2S + SO2 =" 3S" + 2H2O ��2 +4
(2)H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ����ˡ�
(3)SO2 ��ƿ�ڵ���NaOH��Һ����
��С��ÿ��1�֡�
(2)H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ����ˡ�
(3)SO2 ��ƿ�ڵ���NaOH��Һ����
��С��ÿ��1�֡�
����1�����ݷ�Ӧ����ͼʾ��ÿ2��H2S���ӿ���1��SO2���ӷ�Ӧ������������ԭ�Ӻ�����ˮ���ӣ��ݴ˿�д����ѧ����ʽ��2H2S+SO2=3S+2H2O��
��������Ԫ�صĻ��ϼ���+1�ۣ����������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�������������Ԫ�صĻ��ϼ���-2�ۣ����������Ԫ�صĻ��ϼ�Ϊ+4�ۣ�
��2������ƿ����ˣ�ԭ���� H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ�����
��3����֪��ͬ�����£������������������ķ�����Ŀ��ȣ���ʣ�������Ķ����������壬���ն��������������������������Һ��
�𰸣���1��2H2S+SO2=3S+2H2O��-2��+4
��2��H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ�����
��3������������ƿ�ڵ���NaOH��Һ����
��������Ԫ�صĻ��ϼ���+1�ۣ����������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�������������Ԫ�صĻ��ϼ���-2�ۣ����������Ԫ�صĻ��ϼ�Ϊ+4�ۣ�
��2������ƿ����ˣ�ԭ���� H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ�����
��3����֪��ͬ�����£������������������ķ�����Ŀ��ȣ���ʣ�������Ķ����������壬���ն��������������������������Һ��
�𰸣���1��2H2S+SO2=3S+2H2O��-2��+4
��2��H2S��SO2��Ӧ�����ɹ�̬�����Һ̬��ˮ��ʹƿ�ڵ�ѹǿС��ƿ��Ĵ���ѹ����������ƿ�����
��3������������ƿ�ڵ���NaOH��Һ����

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ