��Ŀ����
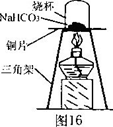
.(10��)��ʽ�Ȼ�����ij�ָ�Ч��ˮ������Ҫ�ɷ֡�ʵ��������������(��Ҫ��Al2O3����������Fe2O3��������������)��ȡ��ʽ�Ȼ����Ĺ������£�

(1)�ܽ�����У���Ҫʹ����������Ϊ15%�����ᣬ����������������Ҫ______mL��30%��Ũ����(�ܶ�ԼΪ1.15g/cm3)��115g����ˮ��
(2)�����ܽ�����������У�������Ӧ�Ļ�ѧ����ʽΪ________________________��
(3)���������۵���Ҫ�����ǣ���ȥ��Һ�е�______��
(4)��CaCO3��ĩ�������ǵ���pH��5����д����pH��ֽ�ⶨ��ҺpH�IJ������ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ��____________________________________���ó�����Һ��pH��

(1)�ܽ�����У���Ҫʹ����������Ϊ15%�����ᣬ����������������Ҫ______mL��30%��Ũ����(�ܶ�ԼΪ1.15g/cm3)��115g����ˮ��
(2)�����ܽ�����������У�������Ӧ�Ļ�ѧ����ʽΪ________________________��
(3)���������۵���Ҫ�����ǣ���ȥ��Һ�е�______��
(4)��CaCO3��ĩ�������ǵ���pH��5����д����pH��ֽ�ⶨ��ҺpH�IJ������ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ��____________________________________���ó�����Һ��pH��
(10��)(1)100(2��) (2)Al2O3+6HCl��2AlCl3+3H2O(2��) Fe2O3+6HCl��2FeCl3+3H2O(2��) (3)������(Fe3+)(2��) (4)������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚ�(2��)
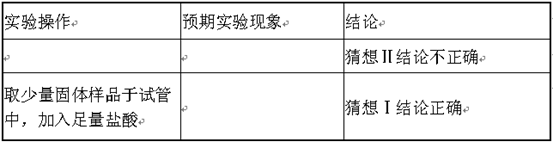
��������1���������ʵ����������������ʵ���������ҹ������֮�Ƚ����
��2�����ý��������������ᷴӦ�����κ�ˮ�Ĺ�����д��ѧ��Ӧ����ʽ��
��3���������������Դ��������Կ����û����Ȼ����е�����
��4������pH��ֽ����ȷ������������pHֵ���в�����
��𣺽⣺��1����Ϊ���ʵ����������������ʵ���������ҹ������֮�ȣ���������Ҫ�����������x
����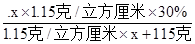 ��100%=15%
��100%=15%
��ã�x=100������
��2�����������������ᷴӦ�����κ�ˮ��������������������Ӧ�����Ȼ�����ˮ������������Ӧ�����Ȼ�����ˮ���ٽ�ϻ�ѧ��Ӧ����ʽ����дҪ����д��ѧ��Ӧ����ʽ��
��3���������ۺ������Դ��������Կ����û����Ȼ�����Һ�е�����ʹ��Һ�в����������ӣ�
��4������pH��ֽ����ȷ���������ǣ�������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pHֵ���в������ʴ�Ϊ����1��100��2��Al2O3+6HCl=2AlCl3+3H2O�� Fe2O3+6HCl=2FeCl3+3H2O
��3�������ӣ�Fe3+��
��4��������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
������������һ�����ʵ��Ʊ������⣬����ͼʾ����֮��ķ�Ӧ�������ص��������̽�ַ������ɣ�
��2�����ý��������������ᷴӦ�����κ�ˮ�Ĺ�����д��ѧ��Ӧ����ʽ��
��3���������������Դ��������Կ����û����Ȼ����е�����
��4������pH��ֽ����ȷ������������pHֵ���в�����
��𣺽⣺��1����Ϊ���ʵ����������������ʵ���������ҹ������֮�ȣ���������Ҫ�����������x
����
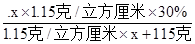 ��100%=15%
��100%=15%��ã�x=100������
��2�����������������ᷴӦ�����κ�ˮ��������������������Ӧ�����Ȼ�����ˮ������������Ӧ�����Ȼ�����ˮ���ٽ�ϻ�ѧ��Ӧ����ʽ����дҪ����д��ѧ��Ӧ����ʽ��
��3���������ۺ������Դ��������Կ����û����Ȼ�����Һ�е�����ʹ��Һ�в����������ӣ�
��4������pH��ֽ����ȷ���������ǣ�������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pHֵ���в������ʴ�Ϊ����1��100��2��Al2O3+6HCl=2AlCl3+3H2O�� Fe2O3+6HCl=2FeCl3+3H2O
��3�������ӣ�Fe3+��
��4��������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
������������һ�����ʵ��Ʊ������⣬����ͼʾ����֮��ķ�Ӧ�������ص��������̽�ַ������ɣ�

��ϰ��ϵ�д�
�����Ŀ
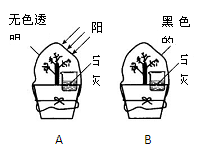
 2MgO + C������þ�Ż�����CO2����𡣵�û���ҵ��й����ܷ���CO2��Ӧ�Ľ��ܡ�����ͬѧ��չ�����Ż��ܷ���CO2������̽����
2MgO + C������þ�Ż�����CO2����𡣵�û���ҵ��й����ܷ���CO2��Ӧ�Ľ��ܡ�����ͬѧ��չ�����Ż��ܷ���CO2������̽����

 ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��
����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾ��