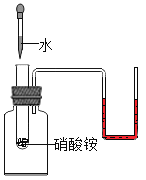
��Ŀ����
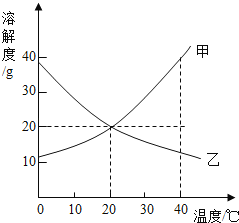
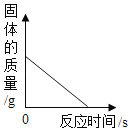
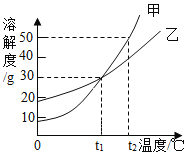
����Ŀ����װ�е���ˮ��A��B��C�����ձ��зֱ����10g��25g��25 g NaNO3���壬�ڲ�ͬ�¶��³���ܽ��������ͼ1��ʾ��
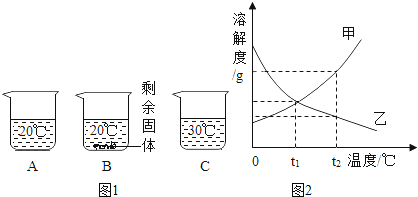
��1���ձ��е���Һһ�����ڱ�����Һ����__________��ѡ�A������B����C����ţ���
��2��ͼ2���ܱ�ʾNaNO3�ܽ�����ߵ���____________����ס����ҡ�����
��3��ҪʹB�ձ���ʣ��Ĺ�������ܽ⣬�ɲ��õķ�����__________��
��4������ͼ2���������ֱ�100g�ס��������ʵı�����Һ��t2�潵�µ�t1�棬��������Һ�ķ�����ȷ����__________������ţ���
A�ס��Ҷ��DZ�����Һ B�����ܼ���������<��
C��Һ��������>�� D����������������>��
���𰸡�B �� �����¶Ȼ���ܼ���ˮ�� BD
��������
��1����ͼ1��֪��20��ʱ��A�ձ��м���10g��ȫ�ܽ⣬�����DZ�����Һ��B�ձ��м���25g��ʣ����壬һ�����ڱ�����Һ��30�����25gȫ���ܽ⣬�����DZ�����Һ��
�ʴ�Ϊ��B��
��2����ͼ1��֪�������¶ȵ������ܽ�������Ƶ����������ӣ�������ͼ2�У��ܱ�ʾNaNO3�ܽ�����ߵ��Ǽף��ʴ�Ϊ���ף�
��3�������¶ȵ������ܽ�������Ƶ����������ӣ�Ҫʹ�ձ�B��ʣ���������ܽ⣬�ɲ��������¶ȵķ����������Բ�ȡ��ˮ�ķ�����
�ʴ�Ϊ�������¶Ȼ���ܼ���ˮ����
��4��A���ҵ��ܽ�����¶Ƚ��������ʽ����¶ȱ�Ϊ��������Һ���ʴ���
B��t2��ʱ���ܽ�ȴ����ҵ��ܽ�ȣ����Ե������ı�����Һ�������ܼ��������Ǽף��ң������¶��ܼ����������䣻�ʽ�100g�ס��ҵı�����Һ�� t2�潵�µ� t1�棬�ܼ���������ϵ��Ȼ�Ǽף��ң�����ȷ��
C���ֱ�100g�ס��ҵı�����Һ�� t2�潵�µ� t1�棬����������������Һ�������ף��ң��ʴ���
D���������ļס��ұ�����Һ�� t2�潵���� t1��ʱ�����������ʣ��������ܽ��������������������͵� t1��ʱ���ܽ���Ǽ�=�ң��������Ҳ��DZ�����Һ�����������ʵ����������Ǽף��ң���ȷ��
��ѡ��BD��

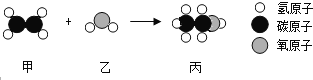
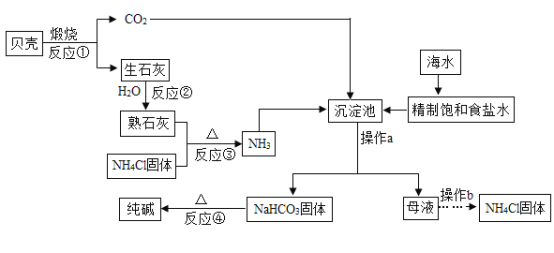
����Ŀ��������������Һ��ͨ�������̼����Ӧ�Ļ�ѧ����ʽΪ________��ʵ��û������������Ҫ֤�����߷����˻�ѧ��Ӧ�����ȡ��ʵ�鷽����_______��
��1����ijͬѧ�ڳ������͵�����������Һ��ͨ�������̼ʱ��������Һ�г����˰�ɫ������ͬѧ�ǶԴ����������Ũ�����Ȥ�����Բ����������ԭ�����̽����
���������ϣ��ٳ����²������ʵ��ܽ��
�������� | �������� | ̼���� | ̼������ |
�ܽ�ȣ���λ��g�� | 109 | 21.5 | 9.6 |
����̼������Һ�в���ͨ�������̼�ɷ������·�Ӧ��Na2CO3+CO2+H2O=2NaHCO3
��̼��������Һ�Լ��ԡ�
��������⣩�����İ�ɫ������ʲô��
���������1������٣�������̼���ƣ�����ڣ�������̼�����ƣ�����ۣ�������________
�����ʵ��1��С�����ɫ�����м������ˮ�ܽ�õ���Һ������Һ�м�������Ȼ�����Һ���۲쵽_____�����ˣ�����Һ�м���________���۲쵽��Һ��Ϊ��ɫ���ó����۲���۳�����
����һ��̽����������������Һ��ͨ�������̼������������ʲô�����йأ�
���������2������٣�����������������Һ��Ũ���йأ�����ڣ�������ͨ���CO2�������Ķ����йء�
�����ʵ��2��
��2�������С�����ʵ����֤������Ƿ������
���ʵ�鷽���ǣ�________
ʵ������________
ʵ����ۣ���NaOH��Һ��Ũ���йء�
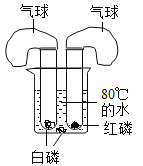
��3����һ��ʵ�鷢����ͨ���CO2�������Ķ���Ҳ�йأ����յó������Ҳ������
����չ�������͵�̼������Һ��ͨ��CO2���壬Ҳ�а�ɫ�������ɡ�
��4���������ɳ�����ԭ��______������ʵ����ۣ�д��һ�ּ��𱥺�̼���ƺ�̼��������Һ�ķ���____
��5��ʵ�鷢�ֳ����£���һ����������������̼������Һ��ͨ�������Ķ�����̼���ܳ��ֳ�������̼������Һ��������������Ӧ����______����������ȷ��0.1%����