��Ŀ����
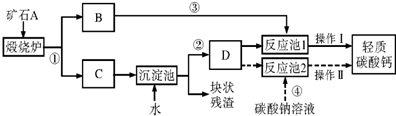
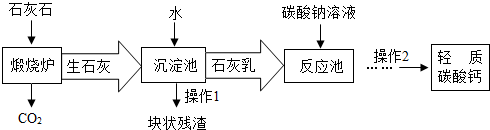
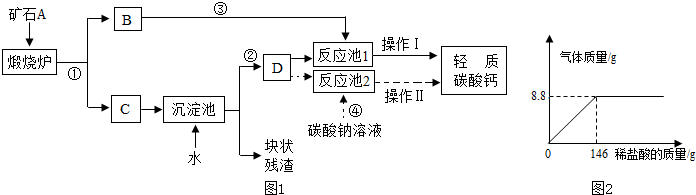
��������Ħ����������̼��ƿ����ÿ�ʯA���Ʊ���ij��ѧ��ȤС�������2��ת�����̣�����ͼ��ʾ��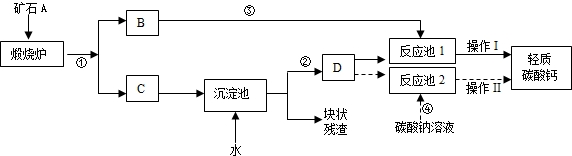
��֪��a��������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2�TCaCO3��+H2O��CaCO3+H2O+CO2�TCa��HCO3��2��
b��̼���������ˮ�����ֽ⣺Ca��HCO3��2 CaCO3��+H2O+CO2����
CaCO3��+H2O+CO2����
c����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ���
����գ�
��1��С�����������̢١��ڡ��ܺͲ��������ƣ���Ϊ�乤�ռ�
��д����Ӧ�ܵĻ�ѧ����ʽ��______�����������______�ȹ���
��2��������̼���ʱ��DΪ______��ѡ�����Һ������Һ��������Һ�����������ǣ�______��
��3��С����Ϊ���̢١��ڡ��ۺͲ���I��С�������Ÿ��ã��������ǣ�______������I����������ȵȹ���
�⣺��1��������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ���Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+Na2CO3�T2NaOH+CaCO3�������ɵ�̼��Ʋ�����ˮ������ͨ�����衢���˵ķ�������Һ�з��������
��2������̼��ƵĿ����dz�ϸС������Һ��Ϊ���ʣ��������Һ���������ƵĿ����dz�ϸС��
��3�����̢١��ڡ��ۺͲ���I��С�������Ÿ��ã�����Ϊ��̼��Ʒֽ����ɵĶ�����̼���Ի������ã�����ʹ�����ɱ����ͣ����������ڽ��ܼ��ţ�
�ʴ�Ϊ����1��Ca��OH��2+Na2CO3�T2NaOH+CaCO3�������衢���ˣ�
��2������Һ���������ƵĿ����dz�ϸС��
��3���������ö�����̼������ʹ�����ɱ����ͣ����������ڽ��ܼ��ţ�
������̼����ֽܷ����������ƣ�����������ˮ��Ӧ�����������ƣ������������������̼��̼���Ʒ�Ӧ����̼��ƣ����ɵ�̼��Ʋ�����ˮ������ͨ�����˵ķ�������Һ�з��������
���������⿼����̼��ơ������ƺ��������Ƶ��ת������ɴ��⣬�����������е�֪ʶ���У�
��2������̼��ƵĿ����dz�ϸС������Һ��Ϊ���ʣ��������Һ���������ƵĿ����dz�ϸС��
��3�����̢١��ڡ��ۺͲ���I��С�������Ÿ��ã�����Ϊ��̼��Ʒֽ����ɵĶ�����̼���Ի������ã�����ʹ�����ɱ����ͣ����������ڽ��ܼ��ţ�
�ʴ�Ϊ����1��Ca��OH��2+Na2CO3�T2NaOH+CaCO3�������衢���ˣ�
��2������Һ���������ƵĿ����dz�ϸС��
��3���������ö�����̼������ʹ�����ɱ����ͣ����������ڽ��ܼ��ţ�
������̼����ֽܷ����������ƣ�����������ˮ��Ӧ�����������ƣ������������������̼��̼���Ʒ�Ӧ����̼��ƣ����ɵ�̼��Ʋ�����ˮ������ͨ�����˵ķ�������Һ�з��������
���������⿼����̼��ơ������ƺ��������Ƶ��ת������ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ

 ��ش��������⣺
��ش��������⣺