��Ŀ����
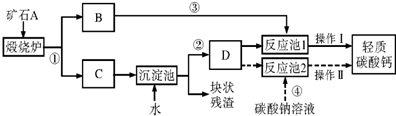
21����������Ħ����������̼��ƿ����ÿ�ʯA���Ʊ���ij��ѧ��ȤС�������2��ת�����̣�����ͼ��ʾ��

���ϣ�a��������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2�TCaCO3��+H2O��CaCO3+H2O+CO2�TCa��HCO3��2��
��
b��̼���������ˮ�����ֽ⣬�������·�Ӧ��Ca��HCO3��2�TCaCO3��+H2O+CO2����
c����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ���飮
������������ת������ͼ�����Ϻش��������⣺
��1��������̼���ʱ��D���õ�������Һ�������ǣ�
��2��С�����������̢١��ڡ��ܺͲ��������ƣ���Ϊ�乤�ռ���д����Ӧ�ܵĻ�ѧ����ʽ��
��3��С����Ϊ���̢١��ڡ��ۺͲ���I��С�������Ÿ��ã��������ǣ�
��һ��
�����

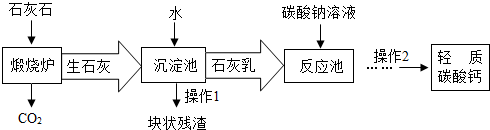
���ϣ�a��������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2�TCaCO3��+H2O��CaCO3+H2O+CO2�TCa��HCO3��2��
��
b��̼���������ˮ�����ֽ⣬�������·�Ӧ��Ca��HCO3��2�TCaCO3��+H2O+CO2����
c����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ���飮
������������ת������ͼ�����Ϻش��������⣺
��1��������̼���ʱ��D���õ�������Һ�������ǣ�
����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵͣ���
����2��С�����������̢١��ڡ��ܺͲ��������ƣ���Ϊ�乤�ռ���д����Ӧ�ܵĻ�ѧ����ʽ��
Ca��OH��2+Na2CO3�TCaCO 3��+2NaOH
����3��С����Ϊ���̢١��ڡ��ۺͲ���I��С�������Ÿ��ã��������ǣ�
��һ��
���ò����Ķ�����̼������̼������Һ�����ã������ɱ����ͣ�
�������
�������Ķ�����̼������ֱ���ŷţ���������ЧӦ�������ϡ����ܼ��š�
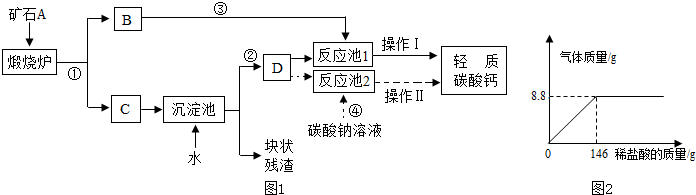
����������1�������������Ƶ��ܽ�Ƚ��з�����
��2���������е������ҳ���Ӧ���������д����ʽ��
��3���������������еķ�Ӧ���������ijɱ���Σ�����з�����
��2���������е������ҳ���Ӧ���������д����ʽ��
��3���������������еķ�Ӧ���������ijɱ���Σ�����з�����
����⣺��1����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ���飬�������Ƶ��ܽ���൱С�����Ƴ�����Һ����ʹ����Ч���൱�ͣ��ʴ�Ϊ������ʯ��ˮ����������Ũ��̫С������Ч�ʺܵͣ�
��2��������C��ˮ��������������D���������ƻ���̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʴ�Ϊ��Ca��OH��2+Na2CO3�TCaCO 3��+2NaOH��
��3������һ��������̶���ʹ�õ��������������ɵĶ�����̼���ȿ��Ա��Ϊ�������Խ��ͳɱ���Ҳ�ܼ�������ЧӦ��
�ʴ�Ϊ��һ�����ò����Ķ�����̼������̼������Һ�����ã������ɱ����ͣ�
������������Ķ�����̼������ֱ���ŷţ���������ЧӦ�������ϡ����ܼ��š���
��2��������C��ˮ��������������D���������ƻ���̼���Ʒ�Ӧ����̼��Ƴ������������ƣ��ʴ�Ϊ��Ca��OH��2+Na2CO3�TCaCO 3��+2NaOH��
��3������һ��������̶���ʹ�õ��������������ɵĶ�����̼���ȿ��Ա��Ϊ�������Խ��ͳɱ���Ҳ�ܼ�������ЧӦ��
�ʴ�Ϊ��һ�����ò����Ķ�����̼������̼������Һ�����ã������ɱ����ͣ�
������������Ķ�����̼������ֱ���ŷţ���������ЧӦ�������ϡ����ܼ��š���
�������ڽ������ʱ�����ȷ�������ͼ�и����Լ������úͷ�Ӧ��������ijɷ֣�Ȼ����ѧ����֪ʶ���з�����

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ
 ��ش��������⣺
��ش��������⣺