��Ŀ����
��12�֣�ͬѧ������ͼ��ʾ������װ��̽����ȡ����ķ�������ش��������⣺

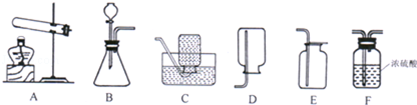
��1��ͼ������B������Ϊ ��
��2��װ��KClO3�Ʊ�O2�ķ���װ�ã���ͼ�л�ȱ�ٵIJ��������� ��д���ƣ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͼ�е���װ��F������ʵ�����Ʊ�O2����Ӧ��ѧ����ʽΪ ��
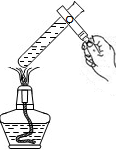
��װ���ڲ����ϵ��ŵ��� ��G��ҽ����Һ�۲��Һ�����ĵκ������������͵�O2����װ�ã�������Ӧ�� (��ѡ��a����b��)���룬�κ���װ���Լ��� ��
��4������Hװ������֤������̼��������ʣ�Ҫ֤��������̼�����������ܹ�������Ӧ�������Լ���˳�����ȼ� �ټ� ������ı仯�� ��

��1��ͼ������B������Ϊ ��
��2��װ��KClO3�Ʊ�O2�ķ���װ�ã���ͼ�л�ȱ�ٵIJ��������� ��д���ƣ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͼ�е���װ��F������ʵ�����Ʊ�O2����Ӧ��ѧ����ʽΪ ��
��װ���ڲ����ϵ��ŵ��� ��G��ҽ����Һ�۲��Һ�����ĵκ������������͵�O2����װ�ã�������Ӧ�� (��ѡ��a����b��)���룬�κ���װ���Լ��� ��
��4������Hװ������֤������̼��������ʣ�Ҫ֤��������̼�����������ܹ�������Ӧ�������Լ���˳�����ȼ� �ټ� ������ı仯�� ��
��1������©�� ��2���ƾ��� 2KClO3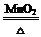 2KCl + 3O2��
2KCl + 3O2��
��3��2H2O2 2H2O+O2�� ���Կ���Һ������������Ʒ�Ӧ���� b Ũ����
2H2O+O2�� ���Կ���Һ������������Ʒ�Ӧ���� b Ũ����
��4��NaOH��Һ������������Һ�� ϡ���� �����ͺ���С
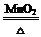 2KCl + 3O2��
2KCl + 3O2�� ��3��2H2O2
 2H2O+O2�� ���Կ���Һ������������Ʒ�Ӧ���� b Ũ����
2H2O+O2�� ���Կ���Һ������������Ʒ�Ӧ���� b Ũ������4��NaOH��Һ������������Һ�� ϡ���� �����ͺ���С
�����������1������������ʶ��
��2����KClO3�Ʊ�O2����Ӧ�����Ǽ��ȣ���װ�䷢��װ�û�ȱ�ٵIJ��������Ǿƾ��ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2KClO3
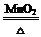 2KCl + 3O2��
2KCl + 3O2����3����װ��F������ʵ�����Ʊ�O2��˵�����ڳ����¹����Һ�巴Ӧ������Ӧ�ǹ���������Һ�Ͷ������̷�Ӧ���ʷ�Ӧ��ѧ����ʽΪ��2H2O2
 2H2O+O2������װ���ڲ����ϵ��ŵ��ǣ����Կ���Һ������������Ʒ�Ӧ���ʣ�G��ҽ����Һ�۲��Һ�����ĵκ������������͵�O2����װ�ã�������Ӧ��b���룬�κ���װ���Լ���Ũ����
2H2O+O2������װ���ڲ����ϵ��ŵ��ǣ����Կ���Һ������������Ʒ�Ӧ���ʣ�G��ҽ����Һ�۲��Һ�����ĵκ������������͵�O2����װ�ã�������Ӧ��b���룬�κ���װ���Լ���Ũ������4��Ҫ֤��������̼�����������ܹ�������Ӧ�����ڶ�����̼���������Ʒ�Ӧ�����ʿ�ͨ���������Ƿ�Ӧ���������̼���ƣ����Բ��õ�ҩƷϡ���ᣬ�ʼ����Լ���˳�����ȼ�NaOH��Һ������������Һ���ټ�ϡ���ᣬ����ı仯�������ͺ���С

��ϰ��ϵ�д�
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�
�����Ŀ