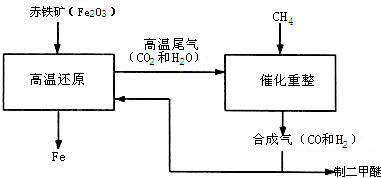
��Ŀ����
Ϊ�˲ⶨ�������ƺ�̼���ƹ���������̼���Ƶ������������ס�����λͬѧ�ֱ���������µ�ʵ�鷽��������ͬѧ�ķ����ǣ���m����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ�����ˡ�ϴ�ӡ���ɣ��Ƶù���n�ˣ���1���������̼���Ƶ���������Ϊ ����m��n��ʾ������ͬѧϴ�ӳ����ľ�������� ��2�������ӡ������Ӷ�����ʹ̼������ӳ�����ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ���ǣ�������ͬѧ�ķ�����ͼ��ʾ��

��1��������ͬѧ��ʵ��װ��ͼ��������ÿ��ʵ���У���ɲⶨ��������Ҫ���� �β��������ո�ͬѧ�ķ������вⶨ�������ʵ��������ڽϴ��������Ϊ��Ҫԭ������ǣ���д��������
��2����ͬѧ�ķ�����װ���д���һ����ȱ�ݣ���������Ľ��ķ��������������������ػ�ͼ���� ��
���𰸡����������������̼���Ƶ���������= %������������Ϊmg��̼���Ƶ������ɸ���̼�ᱵ������ng������������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ�������������
%������������Ϊmg��̼���Ƶ������ɸ���̼�ᱵ������ng������������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ�������������
��������ͬѧ��װ�ý���ʵ��ʱҪ�������²�����������ʯ�ҵ�����������װ�á���һ��������Ʒ�м���������ϡ���ᡢ��Ӧ�����������ʯ�ҵ��������÷�����û�г�ȥװ���п����е�ˮ�����Ͷ�����̼����ʯ��Ҳ��������������е�ˮ�Ͷ�����̼��
����⣺����1����̼���Ƶ�����Ϊx
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197
x ng

x=
�������̼���Ƶ���������= ×100%=
×100%= % �ʴ�Ϊ��
% �ʴ�Ϊ�� %
%
��ͬѧϴ�ӳ����ľ�������ǣ�������м�������ˮ��Ȼ����ˣ��ظ�2-3��
��2�������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ������������ʴ�Ϊ����Ca��OH��2w����ˮ������Ӱ�������������BaCl2��CaCl2����Է������������ij��������������С
����1��ʵ��ʱҪ�������²�����������ʯ�ҵ�����������װ�á���һ��������Ʒ�м���������ϡ���ᡢ��Ӧ�����������ʯ�ҵ��������ʴ�Ϊ��4 ��װ����ԭ�еĿ����еĶ�����̼Ҳ����ʯ�����գ��ڷ�Ӧ�����Ķ�����̼δ����ȫ���գ��ۿ����е�ˮ�Ͷ�����̼Ҳ����ʯ�����բܼ��������������㣬δ�ܽ�̼������ȫ��Ӧ��װ�õ������Բ���
��2���÷�����û�г�ȥװ���п����е�ˮ�����Ͷ�����̼����ʯ��Ҳ��������������е�ˮ�Ͷ�����̼���ʴ�Ϊ��������ķ���װ��ǰ���װͨ������������Һ��װ�ã��ڼ�ʯ��װ�ú�������һ����ʯ��װ�ã��Է����տ����е�ˮ�Ͷ�����̼��
�����������鷴Ӧ���ɵ�ˮ�Ͷ�����̼��ʵ��ʱ��һ��Ҫ�ų������е�ˮ�Ͷ�����̼�ĸ��ţ�
 %������������Ϊmg��̼���Ƶ������ɸ���̼�ᱵ������ng������������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ�������������
%������������Ϊmg��̼���Ƶ������ɸ���̼�ᱵ������ng������������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ���������������������ͬѧ��װ�ý���ʵ��ʱҪ�������²�����������ʯ�ҵ�����������װ�á���һ��������Ʒ�м���������ϡ���ᡢ��Ӧ�����������ʯ�ҵ��������÷�����û�г�ȥװ���п����е�ˮ�����Ͷ�����̼����ʯ��Ҳ��������������е�ˮ�Ͷ�����̼��
����⣺����1����̼���Ƶ�����Ϊx
Na2CO3+BaCl2=BaCO3��+2NaCl
106 197
x ng

x=

�������̼���Ƶ���������=
 ×100%=
×100%= % �ʴ�Ϊ��
% �ʴ�Ϊ�� %
%��ͬѧϴ�ӳ����ľ�������ǣ�������м�������ˮ��Ȼ����ˣ��ظ�2-3��
��2�������Ȼ��ƵĻ�����Ӧ���ɵ�������������ˮ����ʹ������������ʴ�Ϊ����Ca��OH��2w����ˮ������Ӱ�������������BaCl2��CaCl2����Է������������ij��������������С
����1��ʵ��ʱҪ�������²�����������ʯ�ҵ�����������װ�á���һ��������Ʒ�м���������ϡ���ᡢ��Ӧ�����������ʯ�ҵ��������ʴ�Ϊ��4 ��װ����ԭ�еĿ����еĶ�����̼Ҳ����ʯ�����գ��ڷ�Ӧ�����Ķ�����̼δ����ȫ���գ��ۿ����е�ˮ�Ͷ�����̼Ҳ����ʯ�����բܼ��������������㣬δ�ܽ�̼������ȫ��Ӧ��װ�õ������Բ���
��2���÷�����û�г�ȥװ���п����е�ˮ�����Ͷ�����̼����ʯ��Ҳ��������������е�ˮ�Ͷ�����̼���ʴ�Ϊ��������ķ���װ��ǰ���װͨ������������Һ��װ�ã��ڼ�ʯ��װ�ú�������һ����ʯ��װ�ã��Է����տ����е�ˮ�Ͷ�����̼��
�����������鷴Ӧ���ɵ�ˮ�Ͷ�����̼��ʵ��ʱ��һ��Ҫ�ų������е�ˮ�Ͷ�����̼�ĸ��ţ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
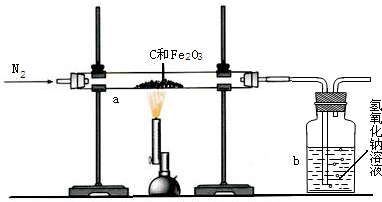
ij��ѧ��ȤС��ͬѧΪ�˲ⶨ������̼��������Ԫ�ص������ȣ��������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȳ�������װ�â��װ�â����������ͼʾ����װ�ã���ȼ����һ��ʱ�������Ϩ���ٷֱ��������װ�â��װ�â��������ʵ���������±���

�������ͼװ�ú����ݻش����⣺
��1��װ�â���Ũ����������� ��
װ�â����������ƺ������ƹ���������� ��
��2�����ϱ����ݿ�֪����Ӧ������ˮ �ˣ�������̼ �ˣ�
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ ��
��4��װ�â��װ�â��ܷ�Ե��� ����ܡ����ܡ�����ԭ���� ��
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ� ��

| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
��1��װ�â���Ũ�����������
װ�â����������ƺ������ƹ����������
��2�����ϱ����ݿ�֪����Ӧ������ˮ
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ
��4��װ�â��װ�â��ܷ�Ե���
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ�
ij��ѧ��ȤС��ͬѧΪ�˲ⶨ������̼��������Ԫ�ص������ȣ��������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȳ�������װ�â��װ�â����������ͼʾ����װ�ã���ȼ����һ��ʱ�������Ϩ���ٷֱ��������װ�â��װ�â��������ʵ���������±���

| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
��1��װ�â���Ũ�����������________��
װ�â����������ƺ������ƹ����������________��
��2�����ϱ����ݿ�֪����Ӧ������ˮ________�ˣ�������̼________�ˣ�
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ________��
��4��װ�â��װ�â��ܷ�Ե���________����ܡ����ܡ�����ԭ����________��
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ�________��
ij��ѧ��ȤС��ͬѧΪ�˲ⶨ������̼��������Ԫ�ص������ȣ��������ͼ��ʾ��ʵ�顣ʵ�鲽�����£��ȳ�������װ�â��װ�â����������ͼʾ����װ�ã���ȼ����һ��ʱ�������Ϩ���ٷֱ��������װ�â��װ�â��������ʵ���������±���

���� | װ�â� | װ�â� | |
��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
�������ͼװ�ú����ݻش����⣺
��1��װ�â���Ũ����������� ��
װ�â����������ƺ������ƹ���������� ��
��2�����ϱ����ݿ�֪����Ӧ������ˮ �ˣ�������̼ �ˡ�
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ ��
��4��װ�â��װ�â��ܷ�Ե��� ����ܡ����ܡ�����ԭ����
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ�
��2007?�ζ�����ģ��ij��ѧ��ȤС��ͬѧΪ�˲ⶨ������̼��������Ԫ�ص������ȣ��������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȳ�������װ�â��װ�â����������ͼʾ����װ�ã���ȼ����һ��ʱ�������Ϩ���ٷֱ��������װ�â��װ�â��������ʵ���������±���

�������ͼװ�ú����ݻش����⣺
��1��װ�â���Ũ�����������______��
װ�â����������ƺ������ƹ����������______��
��2�����ϱ����ݿ�֪����Ӧ������ˮ______�ˣ�������̼______�ˣ�
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ______��
��4��װ�â��װ�â��ܷ�Ե���______����ܡ����ܡ�����ԭ����______��
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ�______��

| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
��1��װ�â���Ũ�����������______��
װ�â����������ƺ������ƹ����������______��
��2�����ϱ����ݿ�֪����Ӧ������ˮ______�ˣ�������̼______�ˣ�
��3���ɸ�ʵ�����ݼ��㣬������̼Ԫ�غ���Ԫ�ص�������Ϊ______��
��4��װ�â��װ�â��ܷ�Ե���______����ܡ����ܡ�����ԭ����______��
��5����С��ͬѧ����װ�â��װ�â����ӵ�����������������ٵ���������������ѧ����֪ʶ�������ǵ��ɻ�______��