��Ŀ����
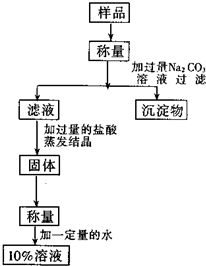 �ú��������Ȼ��Ƶ��Ȼ��ƹ����������ʵ���������Ϊ10%���Ȼ�����Һ�������ͼ��ʾ������������ش�
�ú��������Ȼ��Ƶ��Ȼ��ƹ����������ʵ���������Ϊ10%���Ȼ�����Һ�������ͼ��ʾ������������ش���1����������ʱ����ƽ���̷�
����
����
�����̷�����
����
����2������ʱ��©���¶˹ܿ�Ҫ����
�ձ��ڱ�
�ձ��ڱ�
��©�����Һ��Ҫ������ֽ��Ե
������ֽ��Ե
����3�����������У�Һ�����
������
������
�м��ȣ��������϶ྦྷ��
�϶ྦྷ��
ʱֹͣ����4���û�ѧ����ʽ��ʾ
�ټӹ�����Na2CO3��Һ
Na2CO3+CaCl2�TCaCO3��+2NaCl
Na2CO3+CaCl2�TCaCO3��+2NaCl
���ڼӹ���������
Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
����5������10%���Ȼ�����Һ50g�����ˮ
45
45
g��������ҺʱӦ���ձ�
�ձ�
�н��У���������1��������ƽ��ʹ��ע���������
��2�����ݹ���һ��������������
��3������������������
��4��������д��ѧ����ʽ��ԭ����д����ʽ
��5�������������������ļ����ʽ���ˮ������
��2�����ݹ���һ��������������
��3������������������
��4��������д��ѧ����ʽ��ԭ����д����ʽ
��5�������������������ļ����ʽ���ˮ������
����⣺��1������ƽ������������ʱ���������룬������̷� ���Σ����̷� ���룮
��2������ʱ��©���¶˹ܿ�Ҫ���� �ձ��ڱڣ���ֹҺ�ηɽ���©�����Һ��Ҫ ������ֽ��Ե����ֹҺ�����ֽ��©���ڱ����£�
��3�����������У�Һ����� �������м��ȣ������� �϶ྦྷ��ʱֹͣ���ȣ������������ɣ�
��4���ٴ����к����Ȼ��ƣ�����̼������Һ���Ȼ�����̼������Һ��Ӧ����̼��Ƴ��� ���Ȼ��ƣ���ѧ����ʽΪ
Na2CO3+CaCl2�TCaCO3��+2NaCl��
��������Һ�к��й�����̼���ƣ��ӹ����������ȥ̼���ƣ���ѧ����ʽΪ Na2CO3+2HCl�T2NaCl+H2O+CO2����
��5������10%���Ȼ�����Һ50g�����ˮ������Ϊ50g����1-10%��=45g��������ҺʱӦ�� �ձ��н��У�
�ʴ�Ϊ����1�����Σ����룮
��2���ձ��ڱڣ�������ֽ��Ե��
��3�������� �϶ྦྷ��
��4����Na2CO3+CaCl2�TCaCO3��+2NaCl��
��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��5��45 �ձ�
��2������ʱ��©���¶˹ܿ�Ҫ���� �ձ��ڱڣ���ֹҺ�ηɽ���©�����Һ��Ҫ ������ֽ��Ե����ֹҺ�����ֽ��©���ڱ����£�
��3�����������У�Һ����� �������м��ȣ������� �϶ྦྷ��ʱֹͣ���ȣ������������ɣ�
��4���ٴ����к����Ȼ��ƣ�����̼������Һ���Ȼ�����̼������Һ��Ӧ����̼��Ƴ��� ���Ȼ��ƣ���ѧ����ʽΪ
Na2CO3+CaCl2�TCaCO3��+2NaCl��
��������Һ�к��й�����̼���ƣ��ӹ����������ȥ̼���ƣ���ѧ����ʽΪ Na2CO3+2HCl�T2NaCl+H2O+CO2����
��5������10%���Ȼ�����Һ50g�����ˮ������Ϊ50g����1-10%��=45g��������ҺʱӦ�� �ձ��н��У�
�ʴ�Ϊ����1�����Σ����룮
��2���ձ��ڱڣ�������ֽ��Ե��
��3�������� �϶ྦྷ��
��4����Na2CO3+CaCl2�TCaCO3��+2NaCl��
��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��5��45 �ձ�
���������⿼����ε��ᴿ����Һ�����Ƽ���ѧ����ʽ����д��ע�ػ�����

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
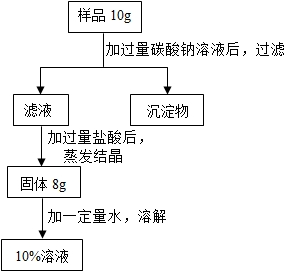 ��2012?��ɽ��һģ���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬������������Ϊ10%���Ȼ�����Һ�������ͼ��ʾ����������������ͼ�ش�
��2012?��ɽ��һģ���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬������������Ϊ10%���Ȼ�����Һ�������ͼ��ʾ����������������ͼ�ش�

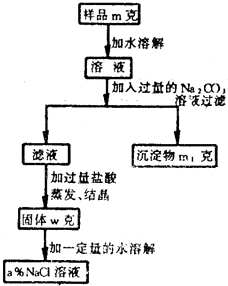 ���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬�������ʵ���������Ϊa%���Ȼ�����Һ�������������ʾ�IJ������������ݷ�������ʾ�ش����и��ʣ�
���ú��������Ȼ��Ƶ��Ȼ��ƹ��壬�������ʵ���������Ϊa%���Ȼ�����Һ�������������ʾ�IJ������������ݷ�������ʾ�ش����и��ʣ�