��Ŀ����
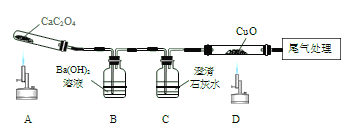
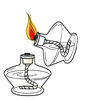
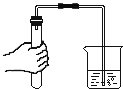
����Ŀ����ѧ��ȤС�黹����������ľ̿��ԭ����ͭ����Ƴ�̽��ʵ��װ������ͼ��ʾ����ش��������⣺
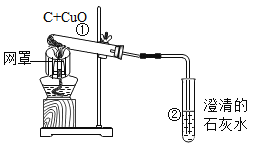
��1���ƾ��Ƽ����ֵ�Ŀ��_____________���տ�ʼԤ�ȣ��Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ����______��
��2���������ȣ��ɹ۲쵽ʯ��ˮ����ǣ���ɫ��ĩ�г��ֺ�ɫ���ʡ�����д����صĻ�ѧ����ʽΪ_____________________��_________________��
��3��ֹͣ����ʱ��Ӧ�Ƚ����ܴ��Թܢ��г��������õ��ɼмн���Ƥ�ܣ����Թܢ���ȴ���ٰ��Թ���ķ�ĩ����������������ԭ����_______________
���𰸡����л���,����¶� �Թ����Ⱥ��ų����� Ca(OH) 2��CO2��CaCO3����H2O 2CuO��C![]() 2Cu��CO2�� ��ֹˮ�����Թ�ը�ѣ��Լ���ֹ���ȵ�ͭ�����������±�����,ʹʵ����������
2Cu��CO2�� ��ֹˮ�����Թ�ը�ѣ��Լ���ֹ���ȵ�ͭ�����������±�����,ʹʵ����������
��������
��1���ƾ��Ƽӵ��ֿ��Լ��л��棬����¶ȣ��Թ����п����տ�ʼ���ȿ�����������Թܢ��������������ݣ����Dz�����ǣ�������л���,����¶ȣ��Թ����Ⱥ��ų��Ŀ���
��2������ʯ��ˮ���������Ϊ������̼��������������̼��ƺ�ˮ�������ķ�ӦΪ��Ca(OH) 2��CO2��CaCO3����H2O����ɫ��ĩ�г��ֺ�ɫ��������Ϊ̼������ͭ��Ӧ������ͭ�Ͷ�����̼�������ķ�ӦΪ��2CuO��C![]() 2Cu��CO2����
2Cu��CO2����
��3��ֹͣ����ʱ��Ӧ�Ƚ����ܴ��Թܢ��г�����Ŀ���Ƿ�ֹ�Թ���ȴ�¶Ƚ��ͣ��Թ��ڿ�����������ѹ���ͣ�ˮ�����룬�Թ���ˮ�����Թ�ը�ѣ��õ��ɼмн���Ƥ�ܣ����Թܢ���ȴ���ٰ��Թ���ķ�ĩ������ԭ���ǣ����Թ�δ��ȴ�����´��ɼУ�����������Թܣ�ͭ�������������ٴ����ɺ�ɫ����ͭ�������ֹˮ�����Թ�ը�ѣ��Լ���ֹ���ȵ�ͭ�����������±�����,ʹʵ���������ԡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ̽������Ļ�ѧ���ʣ�ij��ѧС����������ʵ�飺
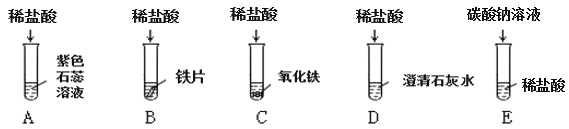
��1��������Ӧ�������������Ϊ_______________������ĸ��ţ���ͬ���������ݲ�������____________��B �Թ�����������Ӧ�Ļ�ѧ����ʽΪ____________��
��2������Ӧ�� D �� E �Թ��еķ�Һ����һ�ྻ���ձ��У��۲쵽�ձ����������ݲ��������а�ɫ�������֡����ձ��еĻ������ˣ��õ���ɫ��������ɫ��Һ��ͬѧ�Ƕ���Һ�����ʵijɷֽ��� ̽����
�������ϵ�֪��CaCl2+Na2CO3=2NaCl+CaCO3����Na2CO3+2HCl=2NaCl+H2O+CO2![]() ��
��
��������⣩��Һ�����ʵijɷ���ʲô��
����������룩����һ��NaCl���������NaCl �� CaCl2 ����������NaCl��Ca(OH)2 �� HCl�������ģ�____________��
�������뽻���������ۣ�ͬѧ��һ����Ϊ����____________�Ǵ���ġ�
��ʵ������ۣ�
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ������Һ���Թ��У��μ�����̼������Һ | ������ | ������ ���� |
ȡ������Һ���Թ��У��μ�����_____________ | ____________ |
����չ��Ǩ�ƣ�ϡ�����ϡ���������ƵĻ�ѧ���ʣ�����Ϊ���ǵ���Һ�ж�����____________��
����Ŀ����ȤС���ͬѧͨ��ʵ��̽����ˮ���ײ�ˮ������Ҫ�ɷ֡�
��������⣩Ӳˮ��к�İ�ɫ���壨ˮ�����к���ʲô���ʣ�
���������ϣ�
��.Ӳˮ�к���һ������ Ca(HCO3)2 �� Mg(HCO3)2������ʱ���������ܽ�ȸ�С�����ʡ� �й����ʵ��ܽ��ԣ�
�� �� | Ca(HCO3)2 | Mg(HCO3)2 | Ca(OH)2 | Mg(OH)2 | CaCO3 | MgCO3 |
�ܽ��� | ���� | ���� | �� | ���� | ���� | �� |
��. Mg(OH)2 �� MgCO3 ���ȷֽ���ֱ����ɶ�Ӧ�����������
�����룩ˮ������Ҫ�ɷ�һ�����в��ܵ�Mg(OH)2 ��________�����ܺ����ܵ�Ca(OH)2��______��
��ʵ�飩
��1������̽����ɫ����ɷ�
����ͬѧȡ��ɫ������������������ˮ����ܽ⣬���ϲ���Һ�еμ�________��Һ��ѡ ����ţ���û�а�ɫ�������ɣ�˵��ˮ������ Ca(OH)2��
A ̼���� B �Ȼ��� C ̼���
����ȡ��ɫ�������ϡ���ᣬ�۲쵽�����ݲ�������ͬѧ��Ϊ������ MgCO3������ͬѧ�������Ľ��ۣ�������__________��
����֪Ca(HCO3)2 ���ȷֽ���̼���������ȷֽ����ƣ���д��Ca(HCO3)2 ���ȷֽ�Ļ�ѧ����ʽ___________________��
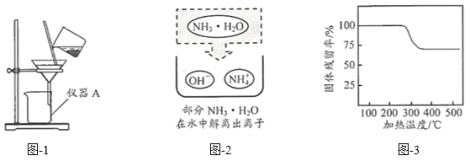
��2��Ϊ��һ��ȷ����ɫ����ijɷ֣���ͬѧ��ȡ 20g ��ɫ���壬�������ʵ�飺

��ʯ�ҵ���Ҫ�ɷ��ǹ��� CaO �� NaOH �Ļ���
����Ӧǰ����ͨһ��ʱ��Ŀ�����Ŀ����______________��
��ʵ���� E װ�õ�������____________________��
����ַ�Ӧ��� C ���� 1.8g��D ������ 6.6g��20g ��ɫ������ Mg(OH)2 ��������___________________g��
�����ۣ��ð�ɫ������������þ��̼��Ƶ�������Ϊ_________��