��Ŀ����
����Ŀ����ͼ��ʾΪ�ⶨˮ����ɵ�ʵ��װ��,���ݹ۲쵽��ʵ������ش�:
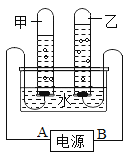
(1)��Դ����Ϊ____(����A������B��)��
(2)���Թ��е��������____����,����ʵ������,�жϸ�������____��
(3)�û���ȼ���Թ��е�����,���Կ���____����,����������____��
(4)������Թ���������ܶ��Ǽ��Թ��������ܶȵ�16��,���Թ���������������Թ������������2��,����Թ������������Թ��������������Ϊ_____��
(5)д�����ˮ��Ӧ�ķ��ű���ʽ:_____��
���𰸡�B �����ǵ�ľ�� ���� ����ɫ ���� 1��8 H2O![]() H2+O2
H2+O2
��������
(1)���ˮʱ���������������������ǡ��������⣬�����һ�����ɴ˿�ȷ����������������ٵ����Թ�����������������֮������B��Ϊ��Դ���������B��
(2)�����ܹ�ʹ�����ǵ�ľ����ȼ�����ô����ǵ�ľ�����飻��������ǵ�ľ����������
(3)���Թ��е��������������ܹ��ڿ�����ȼ�ղ���������ɫ�Ļ��棻����ɫ��������
(4)���ܶȹ�ʽ![]() ��֪�����ˮʱ���缫�ϵõ��������������
��֪�����ˮʱ���缫�ϵõ��������������![]() ���м��㣬����Թ������������Թ��������������
���м��㣬����Թ������������Թ��������������![]() =
=![]() =
=![]() ��2=
��2=![]() �����1��8��
�����1��8��
(5) ���ˮ�����������������䷴Ӧ�ķ��ű���ʽΪ��H2O![]() H2+O2�����H2O
H2+O2�����H2O![]() H2+O2��
H2+O2��

����Ŀ���ᴿ��������ɳ�Ĵ�����Ʒ��ʵ����Ҫ���̺Ͳο��������£�
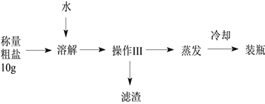
�¶� | 100��ˮ������ܽ��Ȼ��Ƶ����� |
20�� | 36.0 g |
40�� | 36.6 g |
60�� | 37.3 g |
�ٸ�ʵ����������ɳ������ˮ���Ȼ���_____________�����ʽ��з����ᴿ�������������ε���Ҫ������________���������������_________�����в�������������__________��
���ܽ�ʱ������ˮ�ĺ��ʵ���ԼΪ________��ѡ����15������30������60����mL��ѡ���������_______��
A����ʡʵ��ʱ�� B�������е��Ȼ����ܷ���ȫ�ܽ�
C���ձ�����Ͳ�Ĵ�С D�����ٵ��࣬�����ټ�