��Ŀ����
 ����������;�㷺���ش��������⣺
����������;�㷺���ش��������⣺��1�����Ǵ���ʹ�õ��ǺϽ�����Ǵ�����������Ϊ�Ͻ���и����������ܣ���������ȴ���Ӳ��
��2����������ˢ���ᣬĿ������ֹ��������е�������
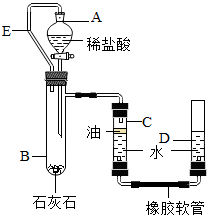
��������ԭ��֮һΪʯ��ʯ��ij��ȤС��Ϊ�˲ⶨij����������ʯ��ʯ��̼��Ƶ�����������������ͼװ�����������ʵ�鷽����
��ʵ��ԭ�������ⶨһ��������ʯ��ʯ������ϡ���ᷴӦ�����Ķ�����̼�ڳ��³�ѹ�µ�����������ܶ����������̼��������Ȼ����ݷ����Ļ�ѧ��Ӧ����ʽ���ʯ��ʯ��̼��Ƶ��������������ʯ��ʯ����̼��Ƶ�����������
��ʵ�鲽�衿��������װ�ò����װ�������ԣ�
�����Թ�B�м���10�˿�ʯA����Һ©���м���������ϡ���ᣬC��D���м���һ������ˮ��C�ܵ�Һ���ϼ�һ ���ͣ�������Һ©��A���Թ�B�ó�����E����������
ʯ��ʯ
�ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ�������A��B�еμ�ϡ�������������ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪV mL�����飬���� B���ܶ�Ϊ��g/L��������������������ɴ����ʯ��ʯ��̼��Ƶ���������
��ʵ�������
��1��С��ͬѧ��Ϊ��ʵ��ǰҪ��ʯ��ʯȫĥ�ɷ�ĩ״������Ϊ����Ŀ���ǣ�
��2��С��ͬѧ��Ϊ��ʵ����������������������A������B����E������Ϊ���û����E������ʵ������������Ӱ�죿
��3������װ�õ�������ʱ����ͼ����װ�ã���C��D������װ��ˮ��Һ����ƽ�����D�ܣ�
��4��ͨ��ʵ��ǰ�����ҩƷ���������ʵ������У���������������㣬Ӧ��;���ỹ������ʵ�飺���жϲ�˵������
��5��ʵ�����ʱͬѧ�Dzŷ������ڴ��ģ�Сΰ��ֲ���ͼӵ���D�ܵ�ˮ���ϣ����һ����Ϊ���ڸô��Ľ��ᵼ�²�õĿ�ʯA��̼��Ƶ�������������������Ϊ�������
��6����ѧ��ʦ�������ǵ�ʵ����ƺ����ǵ���Ʒ����ͺ�����ʶ��̽���������˺ܸߵ����ۣ��������ǽ���������������̼���������Ը��������غ㶨�ɣ��ⷴӦǰ���ձ���ʣ�����ʵ�����֮������ã��������������ձ��н��������µ�ʵ��ⶨ��
| ʵ����� ��Ŀ | ��һ�� | �ڶ��� | ������ |
| ��ȡ��ʯA������/g | 12 | 12 | 15 |
| ����ϡ���������/g | 120 | 150 | 100 |
| ʣ�����ʵ����� | 127.6 | 157.6 | 110.6 |
�ٵ���ȡ��ʯA��ϡ�����������
�ڿ�ʯA��̼��Ƶ����������Ƕ��٣�
������ϡ������������������Ƕ��٣�
���㣺�Ͻ���Ͻ������,ʵ��̽�����ʵ���ɳɷ��Լ�����,�й��������������ļ���,������ʴ�������������,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�������������,�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1���ӺϽ�ȴ��������и�����������ȥ�������
��2������������е�������ˮ������Ӧ�������⣬�����������¶������������ͭ��Һ��Ӧ�������������ͺ�ɫ��ͭȥ�������
��ʵ�������
��1���ӽ�ʯ��ʯȫĥ�ɷ�ĩ״��������ʯ��ʯ��ϡ����ĽӴ����ʹ��Ӧ���ʸ���ȥ�������
��2�������û����E������ϡ����Ҳ��ռ��һ�������ʹ��õĶ�����̼�����ƫ����ʵ����ƫ��ȥ�������
��3�����������Բ��ã�������ͨ��ԭ����������Һ����ƽȥ�������
��4���������;����ᵼ��װ��������©��Ӱ��ʵ����ȥ�������
��5����ʵ���н�ֲ���ͼӵ���C�ܵ�ˮ���ϣ���Ŀ���Ƿ�ֹ�����Ķ�����̼��������ˮ����ˮ������Ӧȥ�������
��6���ٴӱ������ݣ��������ɶ�����̼����������ȣ�������ϡ�����������ʯ��ʯ�����������ȥ�������
�ڸ���12gʯ��ʯ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4gȥ�������
�۸���100gϡ������ȫ��Ӧ���ɶ�����̼������Ϊ4.4gȥ�������
��2������������е�������ˮ������Ӧ�������⣬�����������¶������������ͭ��Һ��Ӧ�������������ͺ�ɫ��ͭȥ�������
��ʵ�������
��1���ӽ�ʯ��ʯȫĥ�ɷ�ĩ״��������ʯ��ʯ��ϡ����ĽӴ����ʹ��Ӧ���ʸ���ȥ�������
��2�������û����E������ϡ����Ҳ��ռ��һ�������ʹ��õĶ�����̼�����ƫ����ʵ����ƫ��ȥ�������
��3�����������Բ��ã�������ͨ��ԭ����������Һ����ƽȥ�������
��4���������;����ᵼ��װ��������©��Ӱ��ʵ����ȥ�������
��5����ʵ���н�ֲ���ͼӵ���C�ܵ�ˮ���ϣ���Ŀ���Ƿ�ֹ�����Ķ�����̼��������ˮ����ˮ������Ӧȥ�������
��6���ٴӱ������ݣ��������ɶ�����̼����������ȣ�������ϡ�����������ʯ��ʯ�����������ȥ�������
�ڸ���12gʯ��ʯ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4gȥ�������
�۸���100gϡ������ȫ��Ӧ���ɶ�����̼������Ϊ4.4gȥ�������
����⣺��1����Ϊ�Ͻ�ȴ��������и����������ܣ��������Ǵ���ʹ�õ��ǺϽ�����Ǵ���������������ȴ���Ӳ�ȴʴ�Ϊ����
��2����������е�������ˮ������Ӧ�������⣬��Ҫ����������������Ƿ����𣬿����������Ϳ��CuSO4��Һ�������������¶������������ͭ��Һ��Ӧ�������������ͺ�ɫ��ͭ���䷴Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TCu+FeSO4���ʴ�Ϊ��Fe+CuSO4�TCu+FeSO4��
��ʵ�������
��1��ʵ��ǰҪ��ʯ��ʯȫĥ�ɷ�ĩ״��������ʯ��ʯ��ϡ����ĽӴ����ʹ��Ӧ���ʸ��죻�ʴ�Ϊ��������ϡ����ĽӴ������
��2�����û����E������ϡ����Ҳ��ռ��һ�������ʹ��õĶ�����̼�����ƫ����ʵ����ƫ�ʴ�Ϊ��ƫ��
��3������װ�õ�������ʱ����ͼ����װ�ã���C��D������װ��ˮ��Һ����ƽ�����D�ܣ�C��D��Һ������̶���Һ�������������ã����������ͨ��ԭ����������Һ����ƽ���ʴ�Ϊ��C��D��Һ������̶���Һ��
��4��ͨ��ʵ��ǰ�����ҩƷ���������ʵ������У���������������㣬�����;����ᵼ��װ��������©��Ӱ��ʵ��������Ӧ����ʵ�飻�ʴ�Ϊ��Ӧ�������飬��Ϊ��;����ᵼ��װ��������©����
��5��ʵ���н�ֲ���ͼӵ���C�ܵ�ˮ���ϣ���Ŀ���Ƿ�ֹ�����Ķ�����̼��������ˮ����ˮ������Ӧ�����²�ö�����̼�����ƫС���Ӷ�ʹ̼��Ƶ���������ƫС���ʴ�Ϊ��ƫС��
��6�����ɱ������ݣ���һ�����ɶ�����̼����Ϊ��12g+120g-127.6g=4.4g���ڶ������ɶ�����̼��������12g+150g-157.6g=4.4g���������ɶ�����̼��������ͬ������ʯ��ʯ����������12g�����ǵڶ��μ������������150g��˵��12ʯ��ʯ�е�̼�����ȫ��Ӧ���ɶ�����̼������Ϊ��4.4g�����������ɶ�����̼������Ϊ��15g+100g-110.6g=4.4g��ʯ��ʯ���������ӵ���15g�����Ƕ�����̼������ȴû�����ӣ�˵��100gϡ������ȫ��Ӧ������4.4g������̼����12gʯ��ʯ��100gϡ����ǡ����ȫ��Ӧ����������Ϊ��12g��100g=3��25���ʴ�Ϊ��3��25��
��������ٵķ�����֪12gʯ��ʯ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4g���裺��ʯA��̼��Ƶ���������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
12g��x 4.4g
=
x=83.3%
�𣺿�ʯA��̼��Ƶ���������Ϊ83.3%
��������ٵķ�����֪100gϡ������ȫ��Ӧ���ɶ�����̼������Ϊ4.4g��������ϡ�����������������y��
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
100g��y 4.4g
=
y=7.3%
��ϡ�����������������Ϊ7.3%
��2����������е�������ˮ������Ӧ�������⣬��Ҫ����������������Ƿ����𣬿����������Ϳ��CuSO4��Һ�������������¶������������ͭ��Һ��Ӧ�������������ͺ�ɫ��ͭ���䷴Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TCu+FeSO4���ʴ�Ϊ��Fe+CuSO4�TCu+FeSO4��
��ʵ�������
��1��ʵ��ǰҪ��ʯ��ʯȫĥ�ɷ�ĩ״��������ʯ��ʯ��ϡ����ĽӴ����ʹ��Ӧ���ʸ��죻�ʴ�Ϊ��������ϡ����ĽӴ������
��2�����û����E������ϡ����Ҳ��ռ��һ�������ʹ��õĶ�����̼�����ƫ����ʵ����ƫ�ʴ�Ϊ��ƫ��
��3������װ�õ�������ʱ����ͼ����װ�ã���C��D������װ��ˮ��Һ����ƽ�����D�ܣ�C��D��Һ������̶���Һ�������������ã����������ͨ��ԭ����������Һ����ƽ���ʴ�Ϊ��C��D��Һ������̶���Һ��
��4��ͨ��ʵ��ǰ�����ҩƷ���������ʵ������У���������������㣬�����;����ᵼ��װ��������©��Ӱ��ʵ��������Ӧ����ʵ�飻�ʴ�Ϊ��Ӧ�������飬��Ϊ��;����ᵼ��װ��������©����
��5��ʵ���н�ֲ���ͼӵ���C�ܵ�ˮ���ϣ���Ŀ���Ƿ�ֹ�����Ķ�����̼��������ˮ����ˮ������Ӧ�����²�ö�����̼�����ƫС���Ӷ�ʹ̼��Ƶ���������ƫС���ʴ�Ϊ��ƫС��
��6�����ɱ������ݣ���һ�����ɶ�����̼����Ϊ��12g+120g-127.6g=4.4g���ڶ������ɶ�����̼��������12g+150g-157.6g=4.4g���������ɶ�����̼��������ͬ������ʯ��ʯ����������12g�����ǵڶ��μ������������150g��˵��12ʯ��ʯ�е�̼�����ȫ��Ӧ���ɶ�����̼������Ϊ��4.4g�����������ɶ�����̼������Ϊ��15g+100g-110.6g=4.4g��ʯ��ʯ���������ӵ���15g�����Ƕ�����̼������ȴû�����ӣ�˵��100gϡ������ȫ��Ӧ������4.4g������̼����12gʯ��ʯ��100gϡ����ǡ����ȫ��Ӧ����������Ϊ��12g��100g=3��25���ʴ�Ϊ��3��25��
��������ٵķ�����֪12gʯ��ʯ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4g���裺��ʯA��̼��Ƶ���������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
12g��x 4.4g
| 100 |
| 44 |
| 12g��x |
| 4.4g |
x=83.3%
�𣺿�ʯA��̼��Ƶ���������Ϊ83.3%
��������ٵķ�����֪100gϡ������ȫ��Ӧ���ɶ�����̼������Ϊ4.4g��������ϡ�����������������y��
CaCO3+2HCl�TCaCl2+H2O+CO2��
73 44
100g��y 4.4g
| 73 |
| 44 |
| 100g��y |
| 4.4g |
y=7.3%
��ϡ�����������������Ϊ7.3%
�����������������ʱ��Ҫ��һ������ͬ������£�ȥ���������ı仯���Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 ��1����ͼ������˿��������ȼ�յ�ʵ��ʱ�����Թ۲쵽�������ǣ���˿
��1����ͼ������˿��������ȼ�յ�ʵ��ʱ�����Թ۲쵽�������ǣ���˿