��Ŀ����
��������ͭ������������ʹ�ñȽϹ㷺�Ľ�����
(1)������Ʒ�У���Ҫ���ý��������Ե��� (�����)��
(2)����Ʒ��������ղ����ø�˿���ϴ�������ƻ������ ��
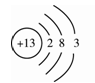
(3)�й��߲˵IJ˵��������������һ��ʱ��������ˡ����ڿ�������ʴ��ʵ���������� �����ʷ�����ѧ��Ӧ�Ľ����
(4)��ʴ������������ұ���DZ���������Դ��һ����Ч;����д����һ����̼�ڸ��������»�ԭ��ʴ�����Ļ�ѧ����ʽ ��
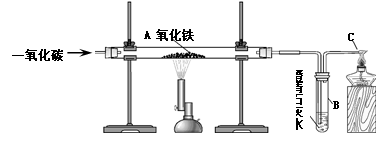
(1)A (2)����Ĥ
(3)�����е�ˮ����������
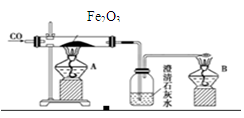
(4)Fe2O3��3CO 2Fe��3CO2
2Fe��3CO2
����

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ